The Many Faces (and Origins) of Adenocarcinoma in the Liver: Pattern Approach to Diagnosis on Small Tissue Samples
Aileen Wee, MB,BS, FRCPath, FRCPA*
Yong Loo Lin School of Medicine**
*Professor and Senior Consultant, Department of Pathology
**National University of Singapore, National University Hospital, 5 Lower Kent Ridge Road,
Singapore, 119074 Email: aileen_wee@nuhs.edu.sg
Correspondence: Aileen Wee, MB,BS, FRCPath, FRCPA
Received: 20 February 2015; Accepted 13 March 2015
ABSTRACT
Focal liver lesions can be solitary or multiple, solid or cystic, congenital or acquired, and range from cysts, hamartomas, hyperplastic nodules and inflammatory entities to tumors and tumor-like lesions. The background liver may be normal or diseased. An algorithmic approach based on pattern recognition and cell profiling is outlined for the diagnosis of focal liver lesions with glandular features in small tissue samples from fine needle aspiration and core needle biopsy. The morphological categories for lesions with glandular phenotypes are (i) glandular (ducts, glands and/or mucin) pattern, including biliary and papillary patterns, (ii) hepatocellular and epithelioid patterns; (iii) mixed epithelioid-glandular (hepatobiliary) pattern; (iv) predominant cell profiles; and (v) cystic pattern. Hepatobiliary entities should be segregated before considering nonhepatobiliary conditions. The main diagnostic issues are to distinguish cystic from solid lesions, primary from metastatic adenocarcinomas, poorly differentiated adenocarcinoma from poorly differentiated hepatocellular carcinoma; and to recognize rare glandular entities, mimics and pitfalls. Assessment of sample adequacy, key cytohistological features, and diagnostic utility of ancillary tests are addressed. Close clinicopathological correlation is mandatory before rendering a final definitive diagnosis.
Keywords: Adenocarcinoma, cholangiocarcinoma, core biopsy, fine needle aspiration cytology, liver, metastases
INTRODUCTION
The anatomical and functional complexity of the liver makes for a highly varied and challenging landscape of pathologies for clinical, radiological and pathological diagnosis. The liver consists of hepatobiliary and other cellular components, receives a dual blood supply, and has a monocyte-macrophage function. Focal liver lesions can be solitary or multiple, solid or cystic, congenital or acquired, and may range from cysts, hamartomas, hyperplastic nodules and infl ammatory entities to tumors and tumor-like lesions.1,2
Three clinicopathological scenarios come to mind when the commonplace adenocarcinoma is addressed in the liver.3, 4 First, the liver is a common depository for secondaries from virtually any part of the body. The majority are metastatic adenocarcinomas which have to be distinguished from primary intrahepatic cholangiocarcinoma (ICC). Second, the liver can undergo cirrhosis with subsequent development of hepatocellular carcinoma (HCC). The pseudoacinar pattern in HCCs and the rare combined hepatocellular-cholangiocarcinoma (CHCC-CC) can be confused with adenocarcinoma. Third, benign neoplastic and nonneoplastic biliary lesions can mimic adenocarcinoma.
Suffice it to say that the differential diagnoses abound when a carcinoma with putative glandular morphology is encountered. It is imperative for prognostic and therapeutic purposes that an accurate cytohistological diagnosis be rendered. This can prove extremely challenging on small tissue samples from fine needle aspiration biopsy (FNAB) and core needle biopsy (CNB).
This article aims to provide an algorithm for the pattern recognition and cell profi ling of focal liver lesions with putative glandular morphology. Intrahepatic cholangiocarcinoma is taken as the reference tumor. Key cytohistological features, diagnostic utility of immunohistochemistry, and diagnostic pitfalls and challenges are addressed. Clinicopathological correlation is mandatory.
CLINICAL PERSPECTIVE
In clinical practice, the initial impression of a liver mass is to investigate for HCC or metastases rather than ICC, unless there is a positive supportive history such as primary sclerosing cholangitis (PSC), chronic biliary tract disease due to hepatobiliary lithiasis and liver flukes such as Clonorchis sinensis or Opisthorchis viverrini, or developmental cystic anomalies.1-5 Metastatic tumors with glandular morphology that are commonly encountered in the liver include those from colorectum, stomach, pancreas, extrahepatic biliary tract, breast, lung, prostate and female genital tract. The background liver may provide important clues. Metastases are rarely found in cirrhotic livers. Pre-existing benign cysts may call attention to themselves with adenocarcinomatous transformation.
Patients may be asymptomatic or present with advanced disease. They fall into several clinical settings:
- Investigation for symptomatology referable to the primary hepatic tumor or liver metastases such as obstructive jaundice, right hypochondrial pain, biliary sepsis or hepatomegaly
- Radiological staging or surveillance for recurrent or metastatic disease in known cancer patients
- Follow-up/surveillance of at-risk patients with pre-existing benign cystic condition or chronic hepatobiliary disorder
- Routine health screening revealing abnormal liver function test profiles, elevated serum tumor markers such as CA19-9 and carcinoembry-onic antigen (CEA), or imaging abnormalities
RADIOLOGICAL PERSPECTIVE
Imaging features of ICC are categorized into mass-forming (MF), periductal infiltrating (PI), and intraductal growth (IG) types.3, 6 The checklist that must be complied with includes exclusion of lesions in other sites, presence of peripheral duct dilatation, delayed enhancement of radiocontrast dye, and hilar lymphadenopathy. Cirrhosis should alert one to the possibility of an HCC or a CHCC-CC. The MF type with irregular borders is the commonest and typically shows arterial and portal venous phase rim-like enhancement and slow diffusion of contrast into the tumor leading to late central enhancement. The poorly enhanced center is due to necrosis and/ or desmoplasia. Capsular retraction is characteristic. The PI and IG types usually present with obstructive jaundice. The PI type tends to simulate benign stricture with thickening and segmental enhancement of the bile ducts. The IG type due to polypoid or papillary growths is uncommon and frequently not detected on imaging as the lesions are usually small.
Liver is the most common intra-abdominal organ for metastases which are often multiple with variable appearances depending on the primary site.3, 7 On ultrasound (US), most metastases appear as hypoechoic masses. On computed tomography (CT), the appearance is highly varied but usually hypodense. Hyperdense metastases can be seen in carcinoid tumor and mucinous adenocarcinoma of colon when they calcify. Some metastases have nonspecific targetoid appearance.
Cystic lesions appear typically anechoic on US, hypodense on CT, and hyperintense on T2-weighted magnetic resonance imaging (MRI). Mucinous cystic neoplasms (MCN) can resemble simple cysts and appear uniformly anechoic on US, with a barely perceptible wall and no internal echoes. Metastases can appear cystic, especially from mucinous adenocarcinomas or post-chemotherapy | necrosis; and so can ICC. Imaging of biliary intraductal papillary neoplasm (IPN) may demonstrate dilated intra-/extrahepatic bile ducts with single or multiple polypoid masses in the biliary tree.
PATHOLOGICAL PERSPECTIVE
Cholangiocytes lining the small-caliber bile duct branches are small and fl attened to cuboidal cells. The epithelium becomes columnar with occasional mucous cells in the larger ducts. It is speculated that ICCs found at these different anatomical locations portray a corresponding morphological topography.8-11 Those mixed hepatobiliary carcinomas (MHBC) portraying hybrid features straddling hepatocytes and cholangiocytes are likely attributed to hepatic stem and progenitor cells in the canals of Hering.12 This phenotypic diversity may be used to diagnostic advantage in distinguishing adenocarcinoma of biliary tract origin from other adenocarcinomas, apart from pancreatic adenocarcinoma since the biliary tract is regarded as an anatomical extension of the pancreas.13 Immunohistochemically, ICCs are characteristically CK7/CK19 positive and CK20 negative.14 However, those closer to the hilum may acquire additional CK20 positivity.
DIAGNOSTIC ALGORITHM
Basic patterns and cell profiles
The approach to the evaluation of small tissue samples begins with the consideration of whether the specimen is from a solid or cystic lesion to enable assessment of adequacy and representativeness. The morphological categories for lesions with glandular phenotypes are (i) glandular (ducts, glands and/or mucin) pattern, including biliary and papillary patterns, (ii) hepatocellular and epithelioid patterns; (iii) mixed epithelioid-glandular (hepatobiliary) pattern; (iv) predominant cell profiles; and (v) cystic pattern (Table 1).3 Hepatobiliary entities should be segregated before considering nonhepatobiliary lesions. Differential diagnoses and diagnostic pitfalls are discussed.
(i) Glandular (ducts, glands and/or mucin) pattern, including biliary and papillary patterns
The most important primary adenocarcinoma in the liver is ICC although metastases are by far more common. Mixed hepatobiliary carcinomas, and MCN and biliary IPN with their associated invasive carcinomas, are addressed in sections (iii) and (v), respectively.
The typical biliary epithelium comprises monolayered honeycomb sheets of cuboidal/colum-nar epithelium. The equidistant, round to oval nuclei are bland with no nucleolus normally. The cell borders are indistinct. Reactive and atypical changes are indicated by the increasing presence of prominent nucleoli, nuclear overlapping, and abrupt nuclear enlargement, leading to a drunken honeycomb appearance. Nuclear pleomorphism with high nuclear-to-cytoplasmic ratio, nuclear membrane irregularities, hyperchromasia, and mitoses appear with increasingly higher grades of biliary intraepithelial neoplasia (BilIN), progressing to in-situ malignant transformation (Fig. 1).15
Intrahepatic cholangiocarcinomas are notoriously heterogeneous even over a small area (Table 2).16-20 Classic ICCs can be graded into well, moderately and poorly differentiated adenocarcinomas (Fig. 2 and 3). Three smear patterns can be recognized on low power scanning, namely: (i) Scanty cell type: This “background rich-tumor cell poor” pattern typically yields sparse, easily overlooked tumor cells entrapped in desmoplastic stroma; (ii) Ductular proliferation type: This “background moderate-tumor cell modest” pattern is a cue for ICC comprising larger tumor cells admixed with plentiful bland, small ductular cells reflecting ductular reaction to chronic hepatic injury;21 and (iii) Usual adenocarcinoma type: This “background poor-tumor cell rich” pattern comprising larger sheets of pleomorphic glandular cells exhibiting cribriform, palisading and ductal appearances, is not characteristic of ICC and requires other biliary identifiers.
Variations and variants of ICC pose challenges to diagnosis on small samples (Fig. 4). Flat sheets, branching structures with peripheral nuclear palisading, papillary structures with fi brovascular cores, 3-dimensional acinar spaces, intracytoplasmic mucin secretion (signet ring cells), and adenosquamous components can be encountered solely or in combination. This striking phenotypic diversity is exemplified even in tissue cores. Histologically, the malignant cells may be arranged in irregular tubular, tubulocystic, nested glandular or papillary configurations, and cordlike, with a predilection for subepithelial zones beneath preexisting bile ducts. “Adenofibromatous” or “pseudoangiomatoid” arrangement reminiscent of von Meyenburg complexes is unique. Transition from relatively bland-looking bile duct cells to dysplastic (BilIN)/malignant cells (carcinoma-in-situ) is a diagnostic clue. Papillary pattern is more likely to indicate metastases or ICC rather than the rare biliary IPN.
It is virtually impossible to distinguish ICC from metastatic pancreaticobiliary adenocarcinomas in the absence of intrahepatic BilIN or carcinoma-in-situ as they have similar immunoprofiles (Fig. 5) (Table 3). Adenocarcinomas with “dirty” necrosis are likely to be colorectal metastases but not exclusively (Fig. 6). Colorectal metastases are notoriously known for their propensity to colonize the existing intrahepatic biliary tree resulting in an intraductal




growth pattern simulating ICC, intraductal type. CK20 versus CK7/CK19 immunoprofiling is helpful. However, the distinction may not be made in cytology material if one is not mindful of such as an occurrence. In the presence of signet ring cell adenocarcinoma, one must first and foremost exclude metastasis from a primary gastric carcinoma. Metas-tases from ductal carcinoma of breast should come to mind if the tumor cells appear rather monomorphic comprising dissociated plasmacytoid cells (Fig. 7). Rarely, an extrahepatic a-fetoprotein-producing adenocarcinoma, for example, from the stomach, may metastasize to the liver.22
Acinar pattern should be distinguished from pseudoacinar structures which are rosette-like arrangements of tumor cells about a space without true luminal brush border or mucin secretion. Common sources of acinar tumors include prostate and pancreas (Fig. 8). Neuroendocrine tumors and HCC often exhibit pseudoacinar pattern (Fig. 9).23-24 The absence of stromal matrix, and in its place the presence of (blood-filled) sinusoidal spaces between pseudoacini, confirms the trabecular-sinusoidal pattern of HCC.
Benign mimics of adenocarcinomas are treacherous. Bile duct hamartoma (von Meyenburg complexes), bile duct adenoma (peribiliary gland hamartoma) and biliary adenofibroma can mimic well-differentiated ICCs.2 The former two conditions are small subcapsular tumorlike nodules detected incidentally during laparoscopy/laparotomy staging for other gastrointestinal malignancies and sent for intraoperative frozen section consultation. Reactive atypia in biliary epithelium and peribiliary glands is often associated with cholangitis, calculous disease, stenting, PSC, and fluke infestation; it has to be distinguished from BilIN and malignant glands. Ductular reaction represents the restoration process at the damaged hepatocellular-stromal interface.21 Ductular epithelial clusters occurring as small dark overlapping nuclei in curved double-cell cords or tramline configurations are often seen in the central scar of focal nodular hyperplasia, and in association with cirrhosis, and large regenerative and dysplastic nodules. They should not be mistaken for cholangiolocellular carcinoma or small cell-type of adenocarcinoma.10, 11 The threshold for diagnosing malignancy should be raised in the event of finding biliary or peribiliary glandular atypia (reactive or degenerative) in the presence of persistent inflammation, such as in inflammatory pseudotumors.25, 26 Degenerative swelling of cells and nuclei coupled with nuclear pyknosis and vacuolated cytoplasm should not be mistaken for pleomorphism with increased nuclear-to-cytoplasmic ratio and hyper-chromasia. Gastrointestinal contamination of endoscopic ultrasound (EUS)-FNA material is also a known pitfall. Arare mimic of(signet ring cell) adenocarcinoma is epithelioid hemangioendothelioma. The individual endothelial tumor cells with intracytoplasmic spaces and eccentric nuclei may or may not contain erythrocytes.27 In cases where the adenocarcinoma is poorly differentiated, distinction from a poorly differentiated HCC or metastases can be challenging. Ancillary tests may or may not be helpful. Correlation with clinical and radiological findings is imperative.
In summary, some features that favor ICCs over metastatic adenocarcinomas apart from those of pancreaticobiliary origin are heterogeneity, insinua-tive invasion at host-tumor interface, accompanying ductular reaction, pseudoangiomatoid appearance, entrapped portal tract structures, and biliary in-situ change. Metastases generally tend to be more mono-morphic.4
(ii) Hepatocellular and epithelioid patterns
Areas with hepatoid/epithelioid features that stain positively for CK7/CK19 are not uncommon in pure ICCs.28 Another consideration is MHBC [see section (iii)].1, 12 A nondescript epithelioid pattern is often encountered in poorly differentiated ICCs (Fig. 3). In the event that one can only arrive at a diagnosis of a poorly differentiated carcinoma, not otherwise specified, the cytohistology report may include the statement that “features are not specific for any primary site; correlate with clinical and radiological findings”.
(iii) Mixed epithelioid-glandular (hepatobiliary) pattern
An ambivalent glandular/epithelioid pattern may represent MHBC.1, 12 In the classic subtype, combined hepatocellular-cholangiocarci-noma (CHCC-CC), one must demonstrate the cyto-morphological and immunohistochemical presence of malignant hepatocytes, adenocarcinoma, and hybrid (transitional) elements (Fig. 10).1,29-33 Sampling error is a distinct pitfall in small tissue samples with the likelihood of the tumor being labeled as HCC, adenocarcinoma or a poorly differentiated carcinoma.
(iv) Predominant cell profiles
The variants of ICCs may display polygonal, oncocytic, small cell, large cell, clear cell, pleomorphic cell, spindle cell or giant cell features.4 Squamous component may be part of an adenosquamous carcinoma commonly encountered in the pancreaticobiliary tract. The differential diagnoses are listed in Table 1.
(v) Cystic pattern
Fine needle aspiration of neoplastic cysts such as MCN and biliary IPN with or without associated malignant transformation, may not yield totally diagnostic material; likewise for malignant foci developing within cystic anomalies.34-39 Targeted placement of the biopsy needle into suspicious areas under radiological guidance may enhance the diagnostic yield (Fig. 11). A cystic appearance can be encountered in mucin-producing ICCs or metastases. Cystically dilated bile ducts can result from obstructive ICCs. Extensive cavitating necrosis from post-therapy effects or otherwise can result in cystic imaging appearances.
Diagnostic utility of immunohistochemistry
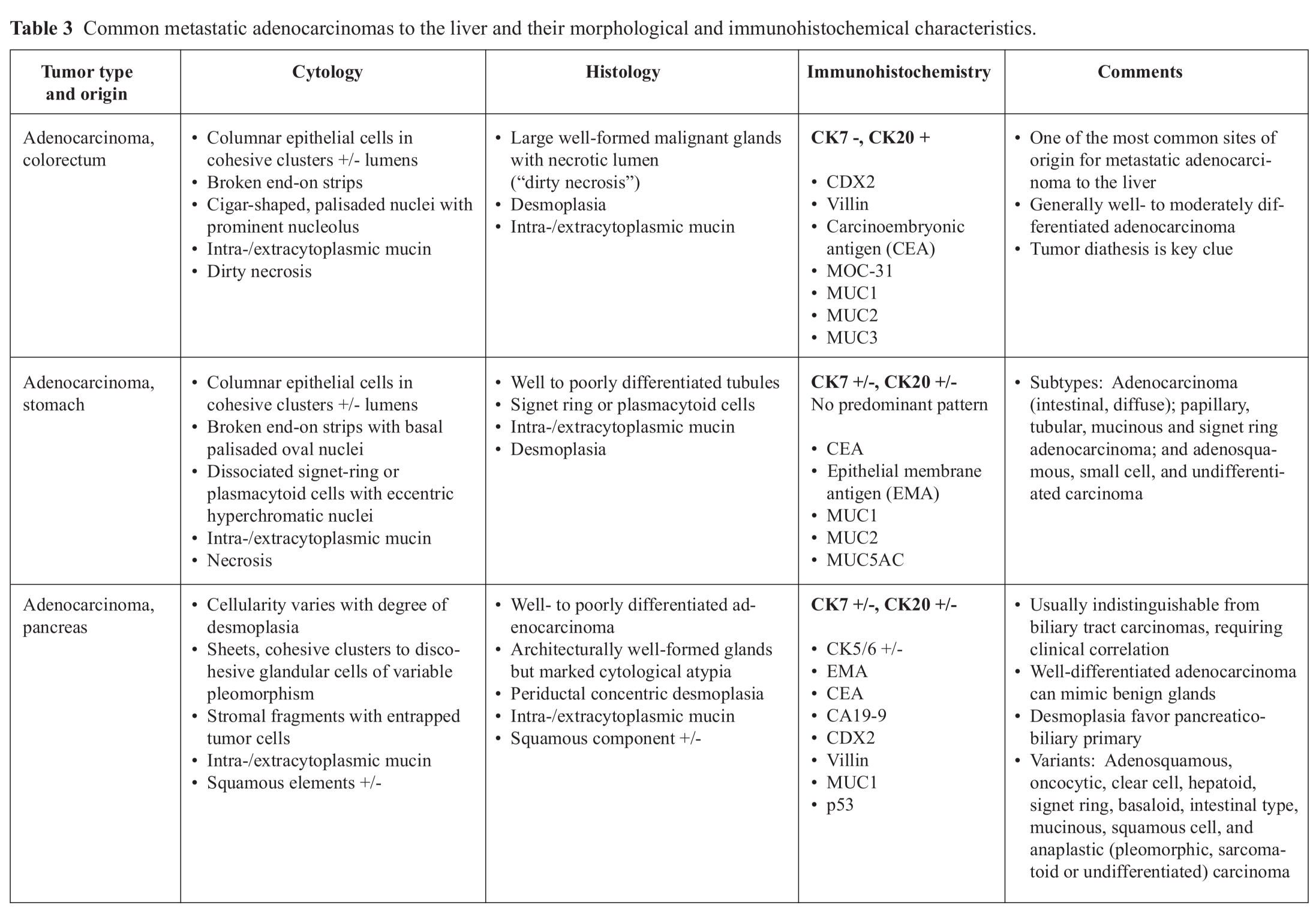
Adenocarcinomas present the most diffi -culty in establishing their site of origin. Metastatic adenocarcinoma is one of the most common tumors encountered in the liver with the majority originating in the gastrointestinal tract, pancreaticobiliary tract, lung, breast and gynecological tract (Table 3). In these times where therapeutic protocols are standardized based on the site of tumor origin and their molecular profiles, it is noted that a diagnosis of adenocarcinoma may not suffice for clinical management. In such cases, clinical information, imaging studies and finally judicious use of immunohisto-chemical stains can help refine the histogenesis and pinpoint the likely site of origin.
The aim of immunohistochemistry in the evaluation of focal liver lesions with glandular features is two-fold, namely (i) to distinguish primary ICC from metastatic adenocarcinomas, and (ii) to define the various subsets of carcinomas in the liver that can be possible differential diagnosis.40-50 The initial immunohistochemical segregation of adenocarcinomas is based on the coordinate expression of CK7 and CK20, listed as follows:14
- CK7 + / CK20 + pattern: Transitional cell carcinoma, pancreatic adenocarcinoma, biliary (hilar) adenocarcinoma, and ovarian mucinous carcinoma
- CK7 + / CK20 — pattern: Lung adenocarcinoma, pancreaticobiliary adenocarcinoma, ductal and lobular breast carcinoma, ovarian carcinoma of serous and endometrioid type, endometrial carcinoma, and (mesothelioma)
- CK7 - / CK20 + pattern: Colorectal adenocarcinoma
- CK7 - / CK20 - pattern: HCC, renal cell carcinoma, prostatic adenocarcinoma, lung squamous cell carcinoma, and lung small cell carcinoma
- No predominant pattern: Gastric adenocarcinoma
Immunohistochemical stains commonly employed include polyclonal carcinoembryonic antigen (pCEA), CD 10, MOC-31, and the cytokeratins.40-43 Polyclonal CEA highlights bile canaliculi but not hepatocytes in normal tissue. Biliary epithelial cells show diffuse cytoplasmic and brush border staining. In high-grade HCC, there is loss of canalicular expression but acquisition of cytoplasmic staining in about 50% cases. This staining pattern poses a challenge in distinguishing HCC from metastatic adenocarcinomas. It is, therefore, important that this stain is utilized keeping in mind the morphological differential diagnosis. CD10 shows similar staining patterns.
MOC-31, also known as epithelial specific antigen, is present in the cytoplasm as well as on cell surface in almost all epithelia, except in most squamous epithelia, hepatocytes, renal proximal tubular cells and gastric parietal cells. It is almost always (80% - 100%) expressed in ICC and metastatic adenocarcinoma from a variety of sites, including but not limited to carcinomas of the pancreas, breast, lung and stomach. This antibody, hence, cannot distinguish ICCs from metastatic adenocarcinomas to the liver. It is, however, either not expressed or is only weakly and focally expressed in HCC.44, 45
Of the cytokeratins, mature hepatocytes stain with CK8 and 18 and CAM5.2 but not with CK7, 19 or 20 or the commonly used CK cocktail, AE1/AE3. Intermediate hepatocytes may show lighter staining with CK7 but not with CK19. CK7 and 19 highlight biliary epithelium (Fig. 12). Focal CK7 positivity can be seen in poorly differentiated HCCs. Hence, the commonly used keratin antibodies (AE1/AE3 and CK7) can be expressed in both HCC and adenocarcinoma, limiting their value. CK7 and 20 are more helpful in determining the primary site once the diagnosis of adenocarcinoma has been established (Fig. 13).
Cytokeratin 19 is expressed in bile duct epithelium but not generally expressed in hepato-cytes. CK19 is expressed in 85% to 100% of ICCs, whereas most HCCs are either negative or show patchy staining, with the exceptiom of fibrolamellar HCC. Mixed hepatobiliary carcinomas may contain a variable proportion of hepatocyte progenitor cells. In such instances, a bi-directional differentiation of neoplastic progenitor cell populations is noted with expression of CK19 as well as CK8 and 18.
SUMMARY
The diagnostic approach is first to evaluate the tissue samples blinded to the clinical and radiological data. Only after a cytomorphological impression is established should one review the available data, before deciding on what special stains and panel of immunohistochemical stains to perform as there is usually limited material. The final diagnosis is based on close clinicopathological correlation. Preparation of cell blocks is very useful for ancillary tests.

Figure 1 Biliary epithelial changes; FNAB and core biopsy of liver.
(A) Biliary intraepithelial neoplasia (BilIN): Large fl at sheet of biliary epithelium begins to lose the orderly honeycomb appearance due to variation in nuclear size and shape and some nuclear overlapping. The nuclei have coarse chromatin and small nucleolus. Papanicolaou, x200.
(B) Biliary adenocarcinoma: Sheets of malignant ductal epithelium show marked disorderly growth with crowding and nuclear overlapping. The variably-shaped nuclei display nuclear membrane irregularities with folds, chromatin clumping, increased nuclear-to-cytoplasmic ratio, and occasional nucleolus. The cytoplasm is pale and vacuolated. Papanicolaou, x200.
(C) BilIN and adenocarcinoma: A resident bile duct (right) exhibits low- to intermediate grade intraepithelial neoplasia with abrupt nuclear atypia and stratification. Malignant glands invade the vicinity which shows desmoplasia and lymphovascular invasion. Hematoxylin & eosin, x100.

Figure 2 Intrahepatic cholangiocarcinoma, well-differentiated; core biopsy of liver with imprint cytology.
(A) Cluster of columnar epithelium with basal nuclei from a malignant gland. May-Grunwald-Giemsa, x400.
(B) Clusters and strips of malignant columnar glandular epithelium with nuclear palisading. The nuclei exhibit pleomorphism, coarse chromatin and small nucleolus. Nonneoplastic hepatocytes are seen at the right lower quadrant. Papanicolaou, x200.
(C) Well-differentiated malignant glands lined by columnar epithelium amid desmoplastic stroma. Hematoxylin & eosin, xl00.
(D) CK7 highlights the malignant glands. Immunostain, x100.
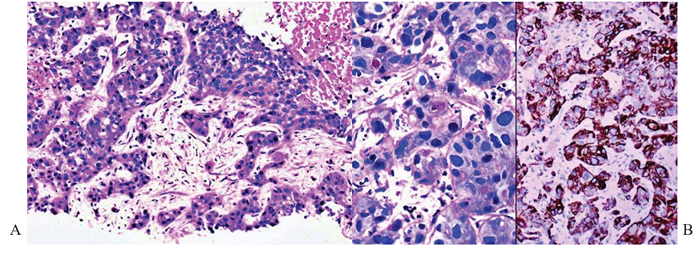
Figure 3 Intrahepatic cholangiocarcinoma, poorly differentiated; core biopsy of liver.
(A) There are sinuous trabeculae of tumor cells exhibiting eosinophilic cytoplasm and round hyperchromatic nuclei. Occasional cytoplasmic vacuoles are discernible. Tumor necrosis is present. Hematoxylin & eosin, x100.
(B) Left panel. Tumor cells contain intracytoplasmic mucin vacuoles. PAS-diastase, x 400. Right panel. CK19 highlights the tumor cells. Immunostain, x200.

Figure 4 Intrahepatic cholangiocarcinoma, variants; core biopsies of liver.
(A) Papillary carcinoma shows fibrovascular cores lined by well-differentiated malignant columnar epithelium. Hematoxylin & eosin, x100.
(B) Signet ring adenocarcinoma cells show eccentric hyperchromatic nuclei and intracytoplasmic mucin vacuoles accompanied by desmoplasia. Hematoxylin & eosin, x200.
Inset: CK7 highlights tumor cells. Immunostain, x 400.
(C) Adenofibromatous variant with pseudoangiomatoid pattern of growth shows intercommunicating spaces lined by largely flattened epithelium and separated by hyalinized collagenous stroma invaded by small malignant glands. Hematoxylin & eosin, xl00.

Figure 5 Adenocarcinomas from pancreas; FNAB and core biopsy of liver metastases.
(A) Sheet of well-differentiated adenocarcinoma cells shows loss of monolayered honeycomb appearance with crowding and nuclear overlapping. Small nucleoli are discernible. Papanicolaou, x200.
(B) Mucinous pools in which float small groups or dissociated malignant glandular cells with enlarged nuclei, high nuclear-to-cytoplasmic ratio and intracytoplasmic mucin vacuoles. May-Grunwald-Giemsa, x200.
(C) Corresponding mucinous adenocarcinoma. Hematoxylin & eosin, x100

Figure 6 Adenocarcinoma from colorectum; FNAB of liver metastases.
Left panel. Tightly cohesive clusters of malignant glands amid striking “dirty necrosis” (tumor diathesis) in the background. Papanicolaou, x40.
Right panel. Broken glands represented by strips of tall malignant columnar epithelium with basal palisading of oval, hyperchromatic nuclei containing distinct nucleolus. Papanicolaou, x400.

Figure 7 Ductal carcinoma from breast; FNAB and core biopsy of liver metastases.
(A) Cohesive clusters of monomorphous, plasmacytoid tumor cells with eccentric round nuclei. May-Grunwald-Giemsa, x200.
(B) Plasmacytoid cells show tendency to dissociation with nuclear streaking artifact. Papanicolaou, x200.
(C) Corresponding histology shows nests of a monomorphic carcinoma with suggestion of glandular differentiation. Note mitotic activity. Hematoxylin & eosin, x200.
Inset: Intense complete membrane staining with c-erbB2. Immunostain. x200.

Figure 8 Acinar adenocarcinoma from prostate; FNAB of liver metastases.
Rosette-like acinar groupings of polygonal well-differentiated tumor cells with vacuolated cytoplasm and regular round nuclei. Complex capillary network holding the tumor cells together like a bunch of grapes. Papanicolaou, x200.

Figure 9 Neuroendocrine carcinoma from pancreas; core biopsy of liver metastases with imprint cytology.
(A) Loosely cohesive aggregate of regular small round tumor cells with suggestion of resetting and nuclear molding. May-Grunwald-Giemsa, x200.
(B) A cluster of tumor cells showing rosette formation with nuclear palisading. May-Grunwald-Giemsa, x400.
(C) Corresponding histology shows nested tumor cells with suggestion of pseudoacinar formation. Hematoxylin & eosin, x200
Inset: Chromogranin highlights the neurosecretory granules in the cytoplasm. Immunostain. x400.

Figure 10 Combined hepatocellular-cholangiocarcinoma; liver resection.
An intimate relationship between well-differentiated adenocarcinoma composed of tubules lined by columnar epithelium and sheets of malignant hepatocytes characterized by well-defined polygonal cells displaying clear cytoplasm and central round nucleus with irregular nuclear contours. Representative sampling is an issue in small tissue samples which may not include both elements which are required for this diagnosis. Hematoxylin & eosin, x100.

Figure 11 Polycystic liver disease with associated invasive adenocarcinoma; FNAB of liver.
Folded sheets of crowded lining epithelial cells with loss of honeycomb appearance. The cells exhibit increasing pleomorphism with variably-shaped nuclei, nuclear membrane irregularities, coarse chromatin, distinct nucleolus, and high nuclear-to-cytoplasmic ratio. Some dissociated tumor cells contain intracytoplasmic vacuoles. The background shows much necrotic debris. Papanicolaou, xl00.

Figure 12 Moderately differentiated adenocarcinoma, primary versus metastasis; core biopsy of liver.
CK7 highlights the adenocarcinoma. Residual ductules and bile ducts are intensely stained. No biliary identifiers are discernible. Immunostain, x100.

Figure 13 Poorly differentiated adenocarcinoma, primary versus metastasis; FNAB and core biopsy of liver.
(A) Cluster of highly pleomorphic malignant cells with high nuclear-to-cytoplasmic ratio, enlarged nuclei and occasional binucleation. May-Grunwald-Giemsa, x400.
(B) Highly pleomorphic tumor cells with tendency to dissociation. The cytoplasm is fragile and vacuolated. Note “poly bag” (left) and necrosis. Papanicolaou, x200.
(C) Corresponding histology shows nests and trabeculae of tumor cells with eosinophilic cytoplasm. Hematoxylin & eosin, x100
(D) The tumor cells are immunoreactive for both CK7 (left panel) and CK20 (right panel). Hilar chol-angiocarcinoma is a possibility. Immunostains, x200.
REFERENCES
1. Bosman FT, Cameiro F, Hruban RH, Theise ND (eds.). World Health Organization Classifi cation of Tumours of the Digestive System. Lyon, IARC Press. 2010.
2. Goodman ZD, Terracciano LM, Wee A. Tumours and tumour-like lesions of the liver. In Burt A, Portmann B, Ferrell L (eds.). Mac-Sween’s Pathology of the Liver, 6th edition. Edinburgh: Churchill Livingstone Elsevier. 2012.
3. Wee A, Sampatanukul P, Jhala N. Cytohistology of Focal Liver Lesions. In: Cytohistology of Small Tissue Samples. Series Eds. K Geisinger & MB Pitman. Cambridge University Press in association with Papanicolaou Society of Cyto-pathology. 2014.
4. Wee A, Pitman MB. Diagnostic Cytology of the Liver. In Odze RD, Goldblum JR (eds.). Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 3rd edition. Philadelphia: Elsevier Saunders. 2015.
5. Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol 2007;23:317-323.
6. Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683-700.
7. Liu PS, Francis IR. Hepatic imaging for metastatic disease. Cancer J 2010;16:93-102.
8. Nakanuma Y, Xu J, Harada K, et al. Pathological spectrum of intrahepatic cholangiocarcinoma arising in non-biliary chronic advanced liver diseases. Pathol Int 2011;61:298-305.
9. Sempoux, C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis 2011;31:49-60.
10. Sempoux C, Fan C, Singh P, et al. Cholangiolo-cellular carcinoma: an innocent-looking malignant liver tumor mimicking ductular reaction. Semin Liver Dis 2012;31:104-110.
11. Kozaka K, Sasaki M, Fujii T, et al. A subgroup of intrahepatic cholangiocarcinoma with an infi ltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: 'bile ductular carcinoma'. Histopathology 2007;51:390-400.
12. Theise ND, Yao JL, Harada K, et al. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology 2003;43:263-271.
13. Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic-counterparts: is the biliary tract an incomplete pancreas? Pathol Int 2010;60:419-429.
14. Chu P, Wu E, Weiss L. Cytokeratin 7 and cy-tokeratin 20 expression in epithelial neoplasms: a survey of435 cases. Mod Pathol 2000;13:962-972.
15. Zen Y, Adsay NV, Bardadin K, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol 2007;20:701-709.
16. Chang KJ. State of the art lecture: endoscopic ultrasound (EUS) and FNA in pancreatico-bil-iary tumors. Endoscopy 2006;38 Suppl 1:S56-60.
17. Eloubeidi MA, Chen VK, Jhala NC, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol 2004;2:209-213.
18. Fritscher-Ravens A, Broering DC, Sriram PV, et al. EUS-guided fine-needle aspiration cytodiag-nosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc 2000;52:534-540.
19. Chaudhary HB, Bhanot P, Logrono R. Phenotypic diversity of intrahepatic and extrahepatic cholangiocarcinoma on aspiration cytology and core needle biopsy: case series and review of the
literature. Cancer 2005;105:220-228.
20. Mohamadnejad M, Dewitt JM, Sherman S, et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc 2011;73:71-78.
21. Sampatanukul P, Leong AS, Kosolbhand P, et al. Proliferating ductules are a diagnostic discriminator for intrahepatic cholangiocarcinoma in FNA biopsies. Diagn Cytopathol 2000;22:359-363.
22. Wee A, Thamboo TP, Thomas A. alpha-Fetoprotein-producing liver carcinomas of primary extrahepatic origin. Acta Cytol 2003;47:799-808.
23. Jackson SB, Williams HJ. Fine-needle aspiration cytology of metastatic carcinoid tumor: report of a case and review of the literature. Diagn Cytopathol 2003;28:49-53.
24. Crapanzano JP. Cytology of low-grade endocrine neoplasms involving liver: a series of 24 specimens, including 4 with hepatoid or glandular features. Diagn Cytopathol 2004;30:31-38.
25. Hosler GA, Steinberg DM, Sheth S, et al. Inflammatory pseudotumor: a diagnostic dilemma in cytopathology. Diagn Cytopathol 2004;31:267-270.
26. Lupovitch A, Chen R, Mishra S. Inflammatory pseudotumor of the liver. Report of the fi ne needle aspiration cytologic findings in a case initially misdiagnosed as malignant. Acta Cytol 1989;33:259-262.
27. Soslow RA, Yin P, Steinberg CR, et al. Cy-topathologic features of hepatic epithelioid hemangioendothelioma. Diagn Cytopathol 1997;17:50-53.
28. Renshaw AA, Haja J, Wilbur DC, et al. Fine-needle aspirates of adenocarcinoma/metastatic carcinoma that resemble hepatocellular carcinoma: correlating cytologic features and performance in the College of American Pathologists Nongynecologic Cytology Program. Arch Pathol Lab Med 2005;129:1217-1221.
29. Jarnagin WR, Weber S, Tickoo SK, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 2002;94:2040-2046.
30. Dusenbery D. Combined hepatocellular-chol-angiocarcinoma: Cytologic findings in four cases. Acta Cytol 1997;41:903-909.
31. Gibbons D, de las Morenas A. Fine needle aspiration diagnosis of combined hepatocellular carcinoma and cholangiocarcinoma. A case report. Acta Cytol 1997;41:1269-1272.
32. Kilpatrick SE, Geisinger KR, Loggie BW, et al. Cytomorphology of combined hepatocellular-cholangiocarcinoma in fine needle aspirates of the liver. A report of two cases. Acta Cytol 1993;37:943-947.
33. Wee A, Nilsson B. Combined hepatocellular-cholangiocarcinoma. Diagnostic challenge in hepatic fine needle aspiration biopsy. Acta Cytol 1999;43:131-138.
34. Pinto MM, Kaye AD. Fine needle aspiration of cystic liver lesions. Cytologic examination and carcinoembryonic antigen assay of cyst contents. Acta Cytol 1989;33:852-856.
35. Horsmans Y, Laka A, Gigot JF, et al. Serum and cystic fl uid CA 19-9 determinations as a diagnostic help in liver cysts of uncertain nature. Liver 1996;16:255-257.
36. Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma-A light microscopic and immunohistochemi-cal study of 70 patients. Am J Surg Pathol 1994;18:1078-1091.
37. Wee A, Nilsson B, Kang JY, et al. Biliary cys-tadenocarcinoma arising in a cystadenoma. Report of a case diagnosed by fine needle aspiration cytology. Acta Cytol 1993;37:966-970.
38. Logrono R, Rampy BA, Adegboyega PA. Fine needle aspiration cytology of hepatobiliary cys-tadenoma with mesenchymal stroma. Cancer 2002;96:37-42.
39. Tsui WM, Lam PW, Mak CK, et al. Fine-needle aspiration cytologic diagnosis of intrahepatic biliary papillomatosis (intraductal papillary tumor): report of three cases and comparative study with cholangiocarcinoma. Diagn Cyto-pathol 2000;22:293-298.
40. Kakar S, Gown AM, Goodman ZD, et al. Best practices in diagnostic immunohistochemistry. Arch Pathol Lab Med 2007;131:1648-1654.
41. Lau SK, Prakash S, Geller SA, et al. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 2002;33:1175-1181.
42. Wang L, Vuolo M, Suhrland MJ, et al. HepPar1, MOC-31, pCEA, mCEA and CD10 for distinguishing hepatocellular carcinoma vs. metastatic adenocarcinoma in liver fine needle aspirates. Acta Cytol 2006;50:257-262.
43. Karabork A, Kaygusuz G, Ekinci C. The best immunohistochemical panel for differentiating hepatocellular carcinoma from metastatic adenocarcinoma. Pathol Res Pract 2010;206:572-577.
44. Niemann TH, Hughes JH, DeYoung BR. MOC-31 aids in the differentiation of metastatic adenocarcinoma from hepatocellular carcinoma. Cancer 1999;87:295-298.
45. Porcell AI, DeYoung BR, Proca DM, et al. Im-munohistochemical analysis of hepatocellular and adenocarcinoma in the liver: MOC31 compares favorably with other putative markers. Mod Pathol 2000;13:773-778.
46. Lei JY, Bourne PA, diSant’Agnese PA, et al. Cytoplasmic staining of TTF-1 in the differential diagnosis of hepatocellular carcinoma vs chol-angiocarcinoma and metastatic carcinoma of the liver. Am J Clin Pathol 2006;125:519-525.
47. Zimmerman RL, Burke MA, Young NA, et al. Diagnostic value of hepatocyte paraffin 1 antibody to discriminate hepatocellular carcinoma from metastatic carcinoma in fine-needle aspiration biopsies of the liver. Cancer (Cancer Cyto-pathology) 2001;93:288-291.
48. Saleh HA, Aulicino M, Zaidi SY, et al. Discriminating hepatocellular carcinoma from metastatic carcinoma on fine-needle aspiration biopsy of the liver: the utility of immunocytochemical panel. Diagn Cytopathol 2009;37:184-190.
49. Gokden M, Shinde A. Recent immunohisto-chemical markers in the differential diagnosis of primary and metastatic carcinomas of the liver. Diagn Cytopathol 2005;33:166-172.
50. Liu H, Shi J, Wilkerson ML, et al. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immu-nomarker for breast and urothelial carcinomas. Am J Clin Pathol 2012;138:57-64.


