Protective effect of angiopoietin 2 on cardiomyocytes against simulated ischaemic injury
Piyanuch Thitiwuthikiat, Duangduan Siriwittayawan and Teonchit Nuamchit*
Department of Cardiothoracic Technology, Faculty of Allied Health Sciences,
Naresuan University, Phitsanulok, Thailand
Correspondence to:
Assistant Professor Dr Teonchit Nuamchit PhD,
Department of Cardiothoracic Technology, Faculty of Allied Health Sciences,
Naresuan University, 99 Moo 9 Phitsanulok-Nakhonsawan Road,
Tha-pho, Muang, Phitsanulok 65000 Thailand.
Telephone: +66 (0) 87 564 4224, +66 (0) 55 966 286 Fax: +66 (0) 55 966 234 Email: teonchitn@nu.ac.th
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Abstract
Angiopoietin 1 (Angpt1) and Angiopoietin 2 (Angpt2) are vascular growth factors that play important roles in vascular angiogenesis and adult vasculature maintenance. Previous studies reported that Angpt1 reduced the size of myocardial infarction in in vivo myocardial infarction experiment in rats, while Angpt2 protein levels increased in myocardial infarction areas. However, it is not clear whether the role of Angpt1 and Angpt2 protect ischemic cardiomyocyte. This research aimed to examine the effects of Angpt1 and Angpt2 on survival of cardiac myoblast cells (H9c2) after ischemic simulation. Initially, H9c2 cells were cultured and pretreated with Angpt1 and Angpt2 at the concentrations of 400, 600 and 800 ng/mL. After the pretreatment, the cells were exposed to simulated ischaemic solution for 90 minutes. Angiopoietin toxicity and cell viability analysis were performed. The results showed that pretreatment with Angpt2 at the concentration of 800 ng/mL significantly increased the cell viability compared to that of the stimulated ischaemic group (p < 0.050). However, there was no significant different in the cell viability between Angpt1 pretreatment and the control group. In summary, pretreatment with Angpt2 works as a cardioprotective molecule in the context of cardiomyocyte ischaemia.
Keywords: angiopoietins; cardioprotective molecule; myocardial ischaemia
Introduction
Ischaemic heart disease is one of a leading cause of death globally. Treatment of myocardial ischaemia is a restoration of myocardial blood flow to the heart muscle or reperfusion. Myocardial reperfusion techniques including percutaneous coronary intervention and antiplatelet and antithrombotic agents has been intensively developed to restoration of myocardial blood flow. However, the protection of myocardial injury after ischaemia is still required. From previous studies, it has been reported that angiopoietin-Tie system plays an important role in vascular angiogenesis, vascular quiescence, and vascular survival. Angiopoietin (Angpt) is a ligand of Tie receptor and consists of four subtypes; Angpt1, Angpt2, Angpt3, and Angpt4. Angpt1 is well elucidated as a vascular protective molecule for vascular stability(1,2). Unlike Angpt1, Angpt2 is more versatile and dynamic functions as a vessel-destabilizing molecule(3). However, the role of the angiopoietin-Tie receptor system in cardiovascular disease is not well known, especially in myocardial damage. Some studies reported that Angpt1 and Angpt2 are involved in pathophysiology of cardiovascular diseases(4). Previous studies showed that plasma Angpt2, but not Angpt1, levels were higher in patients with ST-segment elevation myocardial infarction (STEMI)(5-7). In addition, research data demonstrated that excessive Angpt2 overexpression exacerbated myocardial fibrosis(8,9). However, Dallabrida et al. reported that Angpt2 also stimulated myocardial survival but is less efficiency than Angpt1(10). Thus, the effects of angiopoietin system and its mechanism is not well understood. Cardiac myocyte apoptosis and necrotic myocyte cell death are the results of myocardial ischaemia caused by imbalance of myocardial oxygen supply and demand. Recent study reported myocardial ischaemia-reperfusion induces disbalance of angiopoietins. Angpt1 was likely to protect myocardial ischaemia, while Angpt2 had mysterious effect on cardiac myocytes but the mechanism is still unclear.
There are currently limited data about the effects of angiopoietin and its mechanism on cardiomyocyte survival during ischaemia. The signaling pathways responsible for this phenomenon is needed to be explored. Therefore, this study aimed to examine the effects of Angpt1 and Angpt2 on rat embryonic ventricular myocardial cell line (H9c2) during myocardial ischaemia. The experiments are performed to test the hypothesis that Angpt1 would reduce myocardial damage after ischaemia as well as Angpt2 would aggravate or protect cardiomyocyte subjected to simulated ischaemia.
This research will help to understand the roles of Angpt1 and Angpt2 in myocardial ischaemia and extend the knowledge in development of selective angiopoietin inhibitor for blocking its interaction with their receptor.
Materials and Methods
Chemical and reagents:
Recombinant human angiopoietin-1 protein and recombinant human angiopoietin-2 protein were purchased from R&D system company (Minneapolis, USA). A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (Missouri, USA).
Cell culture:
The rat embryonic ventricular myocardial cell line or H9c2 (ATCC number CRL-1446) were cultured in complete medium; Dulbecco's modified Eagle's medium (DMEM) containing 1500 mg/L of NaHCO2 supplemented with 10% heat-inactivated foetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were incubated with 5% CO2 at 37 °C. Cells were subcultured for subsequent experiments when 80% confluence was reached. For cell toxicity analysis, cells were cultured and divided into 24-well plates at density of 50,000 cells/cm2. After 1 hour of serum deprivation, cells were ready for further experiments.
Experiment protocol:
H9c2 cells at density of 50,000 cells/cm2 in 24-well plates were treated with various concentrations (400, 600 and 800 ng/mL) of Angpt1 and Angpt2 for 1 hour before simulated ischemia. Simulated ischaemia was induced by incubating the cells with a simulated ischaemic solution containing 140 mM NaCl, 6 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5mM HEPES, 10mM 2-deoxy-d-glucose, and 10 mM sodium dithionite(11). Cells cultured in complete medium was used as a control group. Cells were exposed to simulated ischaemic solution at 37 °C in an atmosphere with 5% CO2 for 90 minutes.
Cell viability assay:
In order to determine cell viability of cardiomyocyte with simulated ischaemic condition, MTT assay was performed. After 90 minutes of simulated ischaemia, the solution was removed and cells were washed once with phosphate buffer saline (PBS). MTT solution at the concentration of 0.5 mg/ml was added and incubated at 37 °C for 2 hours. After that, the supernatant was gently removed and dimethyl sulfoxide was used to solubilise the purple formazan crystals. The absorbance of the samples was measured using a microplate reader at the wavelength of 570 nm.
Statistical analysis:
Data are expressed as mean ± standard error of the mean (SEM). All comparisons were analysed using analysis of variance (one-way ANOVA) followed by the Turkey-Post hoc test. The statistical tests were performed using GraphPad Prism version 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The p-value less than 0.050 was considered statistically significant.
Results
Effect of angiopoietins on cell viability:
Cells were treated with three concentrations of Angpt1 and Angpt2 (400, 800 and 1,200 ng/mL) for 1 hour before measuring cell viability using MTT assay. The result showed that there was no significant difference between the number of cells treated with Angpt1 and Angpt2 at every concentrations and that of the control group (Figure 1). Therefore, these nontoxic concentrations of Angpt1 and Angpt2 were chosen to use in the subsequent experiment.
Effect of the simulated ischaemia in different durations on cell viability:
H9c2 cells were subjected to simulated ischaemia with ischaemic solution. Cells were incubated with the ischaemic solution at various durations including 0, 30, 60, 90 and 120 minutes, and MTT assay was performed. Figure 2 shows that the number of cells treated at every duration were significantly lower than that of the control group. In addition, the number of cells treated at 90 and 120 minutes were significantly lower than those of the 30 and 60 minutes of simulated ischaemia. Therefore, the optimal simulated ischaemia duration of 90 minutes was selected for the subsequent experiment.
Effect of angiopoietins on H9c2 cell viability after simulated ischaemia:
H9C2 cells were pretreated with different concentrations (400, 600 and 800 ng/ml) of Angpt1 and Angpt2 for 1 hour. After 90 minutes of simulated ischaemia, MTT assay was performed. Figure 3 shows that the number of cells pretreated with 800 ng/mL of Angpt2 was significantly higher than that of the simulated ischaemic (sI) group, approximately a 20% increase. In addition, at 800 ng/mL of Angpt2 pretreatment, the cell viability was significantly higher than those of the Angpt1 groups.
P
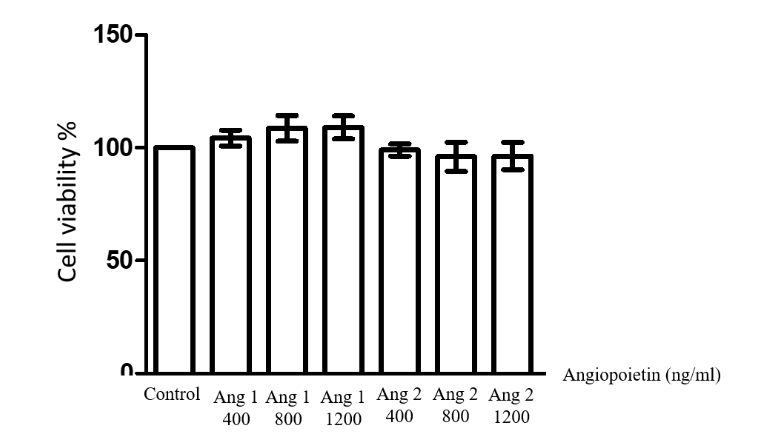
Figure 1 Determination of H9c2 cell viability after treatment with different concentrations of Angpt1 and Angp2 (400, 800 and 1,200 ng/mL). The results are expressed as mean ± standard error. Three experiments with independent cell preparations were performed.
Note: Angpt1 = Angiopoietin1; and Angpt2 = Angiopoietin2
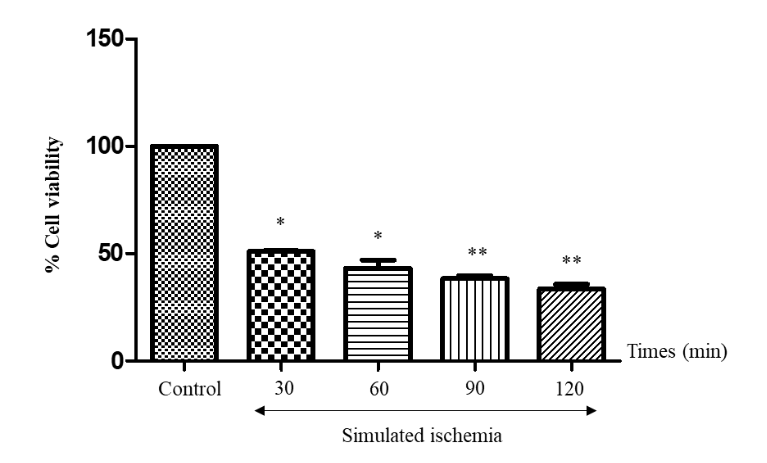
Figure 2 Determination of H9c2 cell viability after simulated ischemia at different durations (30, 60, 90 and 120 minutes). The results are expressed as mean ± standard error. Three experiments with independent cell preparations were performed.
Note: * p < 0.050 versus control group.
** p < 0.050 versus the 30-minutes and 60-minutes exposure group.
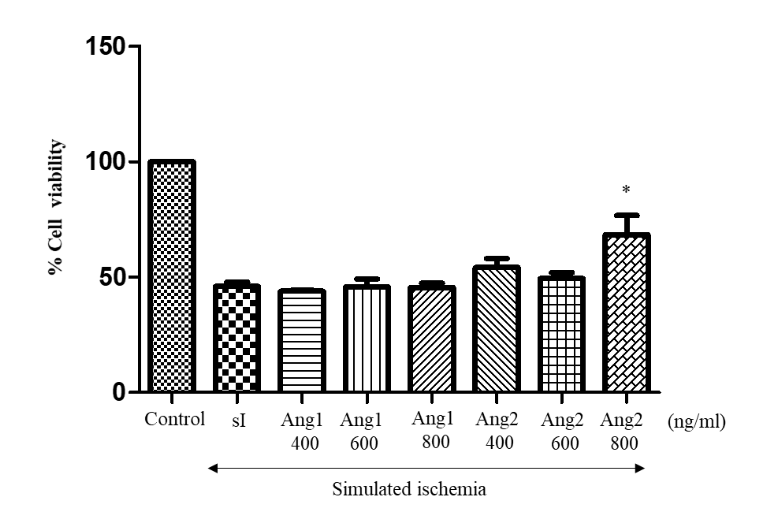
Figure 3 Effects of Angpt1 and Angpt2 on H9c2 cell viability. The results are expressed as mean ± standard error. Three experiments with independent cell preparations were performed.
Note: * p < 0.050 versus the simulated ischaemic group.
Angpt1 = Angiopoietin1; Angpt2 = Angiopoietin2; and sI = Simulated ischaemia
Discussions
The cardiac myocyte mass loss following myocardial ischaemic-reperfusion (I/R) injury remains the leading cause of morbidity and mortality worldwide. Therefore, molecules that can preserve myocyte viability are candidate therapeutic targets to limit myocardial I/R injury. The present study demonstrates that exogenous human recombinant Angpt2 could preserve cardiac myocyte viability in vitro model of simulated ischaemia. The results show that there is significant difference in cell death following simulated ischaemic injury between the untreated group and the cells treated with 800 ng/mL of Angpt2. The data indicates that Angpt2 could suppress simulated ischaemic solution-induced cell death, whereas Angpt1 had no protective effect in this model. Thus, this study reveals that 800 ng/mL of Angpt2 could reduce cardiomyocyte death following simulated ischaemia injury.
The result was consistent with the previous study which reported that 800 ng/mL of Angpt2 could be an apoptosis survival factor for endothelial cells by activation of the phosphatidylinositol 3′-kinase (PI3K)/Akt signal transduction pathway(12). It was also reported that 400 ng/mL of Angpt2 could enhance endothelial cell survival through pro-survival PI3K/Akt pathway(13) and also promote angiogenesis in vitro through the direct activation of Tie2 receptor signaling during prolong exposure or at high concentrations of Angpt2(14). Likewise, Sandhu et al. performed in vivo left coronary artery ligation in rat to induce myocardial ischaemia. They found an increase in Angpt2 and decrease in Angpt1 expression, whereas Tie2 expression remained unchanged(15).
These data indicated the elevation of Angpt2 responded to hypoxia or inflammatory in the ischaemic heart. In turn, Chen et al.(9) suggested that myocardial Tie2 expression was decreased during ischaemia and could be suppressed by high Angpt2 level which was relevant to the studies of Shyu et al.(1) showing the increased Angpt2 expression at the infarct size of rat myocardium. These evidences supported that Angpt2 also increased after ischaemia-reperfusion in the rat ventricular myocardium. However, several studies reported that Angpt2 had a deleterious effect in myocardial I/R injury that predisposed to abnormal vascular remodeling, inflammation, cardiac hypoxia and infarction(8).
Our findings suggest that the physiological role of Angpt2 is more complex than previously recognised. Further studies should elucidate the molecular mechanism responsible for cardioprotective effect of Angpt2 on myocardial survival against ischaemic-reperfusion injury.
Conclusion
In conclusion, the present study demonstrates that human recombinant angiopoietin2 could protect cardiomyocytes against myocardial ischaemic injury in in vitro cytotoxicity model.
Acknowledgment
This study was supported by the Naresuan University Fund (grant no. R2559C008) and National Science and Technology Development Agency Fund (grant no. SCH-NR2015-851).
References
- Shyu KG, Chang CC, Wang BW, Kuan P, Chang H. Increased expression of angiopoietin-2 and Tie2 receptor in a rat model of myocardial ischaemia/reperfusion. Clin Sci (Lond). 2003;105(3):287-94.
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165-77.
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29(8):2011-22.
- Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci U S A. 2003;100(22):12753-8.
- Chen S, Guo L, Cui M, Sun L, Mi L. Dynamic changes in serum angiopoietin-1, angiopoietin-2, and angiopoietin-2/angiopoietin-1 ratio in acute myocardial infarction patients treated with primary percutaneous coronary intervention. Biomarkers. 2012;17(5):441-6.
- Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110(16):2355-60.
- Liu KL, Lin SM, Chang CH, Chen YC, Chu PH. Plasma angiopoietin-1 level, left ventricular ejection fraction, and multivessel disease predict development of 1-year major adverse cardiovascular events in patients with acute ST elevation myocardial infarction - a pilot study. Int J Cardiol. 2015;182:155-60.
- Lee S-J, Lee C-K, Kang S, Park I, Kim YH, Kim SK, et al. Angiopoietin-2 exacerbates cardiac hypoxia and inflammation after myocardial infarction. The Journal of clinical investigation. 2018;128(11):5018-33.
- Chen JX, Zeng H, Reese J, Aschner JL, Meyrick B. Overexpression of angiopoietin-2 impairs myocardial angiogenesis and exacerbates cardiac fibrosis in the diabetic db/db mouse model. Am J Physiol Heart Circ Physiol. 2012;302(4):H1003-12.
- Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96(4):e8-24.
- Yu Y-P, Huang X-M, Fu Y-F. Curcumin protects H9c2 cardiomyocyte against ischemia/reperfusion injury through inactivation of glycogen synthase kinase-3. Int J Clin Exp Pathol. 2016;9(3):3226-32.
- Kim I, Kim J-H, Moon S-O, Kwak HJ, Kim N-G, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000;19(39):4549-52.
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29(8):2011-22.
- Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49(3):659-70.
- Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, et al. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64(1):115-24.


