CD43 Expression in Malignant Melanoma : A Diagnostic Pitfall
Sanya Sukpanichnant
Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Tel/Fax +66 2 411 4260 e-mail: sanya.suk@mahidol.ac.th
Received: 24 September 2014; Accepted 30 October 2014.
ABSTRACT
CD43 is commonly concerned as a marker for hematopoietic cells and, at times, a preferential T-cell marker. However, a number of non-hematologic cancers, mostly carcinoma, can express CD43. Recently, a case of malignant melanoma mimicking malignant lymphoma by cytomorphology was reported to express CD43 as well as melanoma markers but the other hematologic markers [Buehler et al. Diagn Cytopathol 2012;40:619-23]. This is another case report. A 52-year-old man suffered from a rapidly growing left tonsillar mass for one month causing slurred speech. An incisional biopsy showed gray tissue and, beneath the epithelium, a sheet of large tumor cells with distinct nucleolus and somewhat distinct cell borders. Focal infiltration of the epithelium by tumor cells was noted. The first pathologist’s impression was hematologic malignancy, possibly plasmab-lastic lymphoma, as the original workup showed focal and faint CD138 expression but negative keratins and CD45. On consultation, hematologic malignancy and malignant melanoma were considered. The tumor cells express CD43, CD68 (focal), S-100, HMB45, Melan A, and vimentin. The negative markers included CD3, CD20, CD30, CD79a, MUM1, PAX5, kappa, lambda, and CK5/6. The proliferation index was high (70-75%) by Ki-67. Masson Fontana stain showed fine melanin pigment granules in only few tumor cells. In summary, another case report of malignant melanoma with CD43 expression, mimicking malignant lymphoma, raises the awareness of diagnostic pitfall when dealing with undifferentiated tumors showing only CD43 expression among various hematologic markers. A panel for keratins, vimentin, S-100, CD45, CD30, and CD138 will be helpful when dealing with undifferentiated large cell tumor.
Keywords: CD43 • T-cell marker • hematologic malignancy • non-hematologic malignancy • malignant melanoma
*This case report has been presented partly in a poster session in the 12th Japanese-Korean Lymphoreticular-Workshop 2014 in conjunction with the 5th Asian Hematopathology Symposium on January 25, 2014 at Nagoya University Hospital Lecture Hall.
INTRODUCTION
CD43 has been used as a T-cell marker for immunophenotypic study in malignant lymphoma1. At times, CD43 is the only marker suggesting T-cell phenotype in non-Hodgkin lymphoma (NHL). Nevertheless, it is well known about the nonspecific lineage marker of CD43 as it has been used in wide range of hematologic and non-hematologic conditions1-3. So it means that, before concluding T-cell phenotype in any NHL based on CD43 while other T-cell markers are negative, exclusion of other hematopoietic or lymphoid lineages is a must as CD43 has been reported to be expressed on a number of B-cell NHL and non-lymphoid hematopoietic neoplasms.
Recently, CD43 has been found to express in a number of non-hematologic neoplasms2-3. So not only non T-cell hematologic neoplasms but also non-hematologic neoplasms should be considered in differential diagnosis before making a diagnosis of CD43+ only T-cell NHL. Recently, a case of malignant melanoma was reported to express CD434 so this is another report of such a case that created diagnostic difficulty.
CLINICAL HISTORY
A 52-year-old man suffered from a rapidly growing left tonsillar mass for one month causing slurred speech. An incisional biopsy showed gray tissue and, beneath the epithelium, a sheet of large tumor cells with distinct nucleolus and somewhat distinct cell borders. Focal infiltration of the epithelium by tumor cells was noted. The first pathologist’s impression was hematologic malignancy, possibly plasmablastic lymphoma, as the original workup showed focal and faint CD138 expression but negative AE1/AE3 keratins and CD45.
MATERIALS AND METHODS
The provided paraffin-embedded formalin-fixed tissue block of the tonsillar biopsy was used for histopathologic, histochemical, and immunohis-tochemical (immunostaining). Standard hematoxylin & eosin-stained (H&E) slide and PAS-stained slide were performed conventionally5. Immunostaining was performed by Bench-Mark® XT autostainer (Roche Diagnostic). All the primary antibodies were used in dilutions according to the manufacturer specification sheets. Masson Fontana stain was performed according to AFIP laboratory manual5.
RESULTS
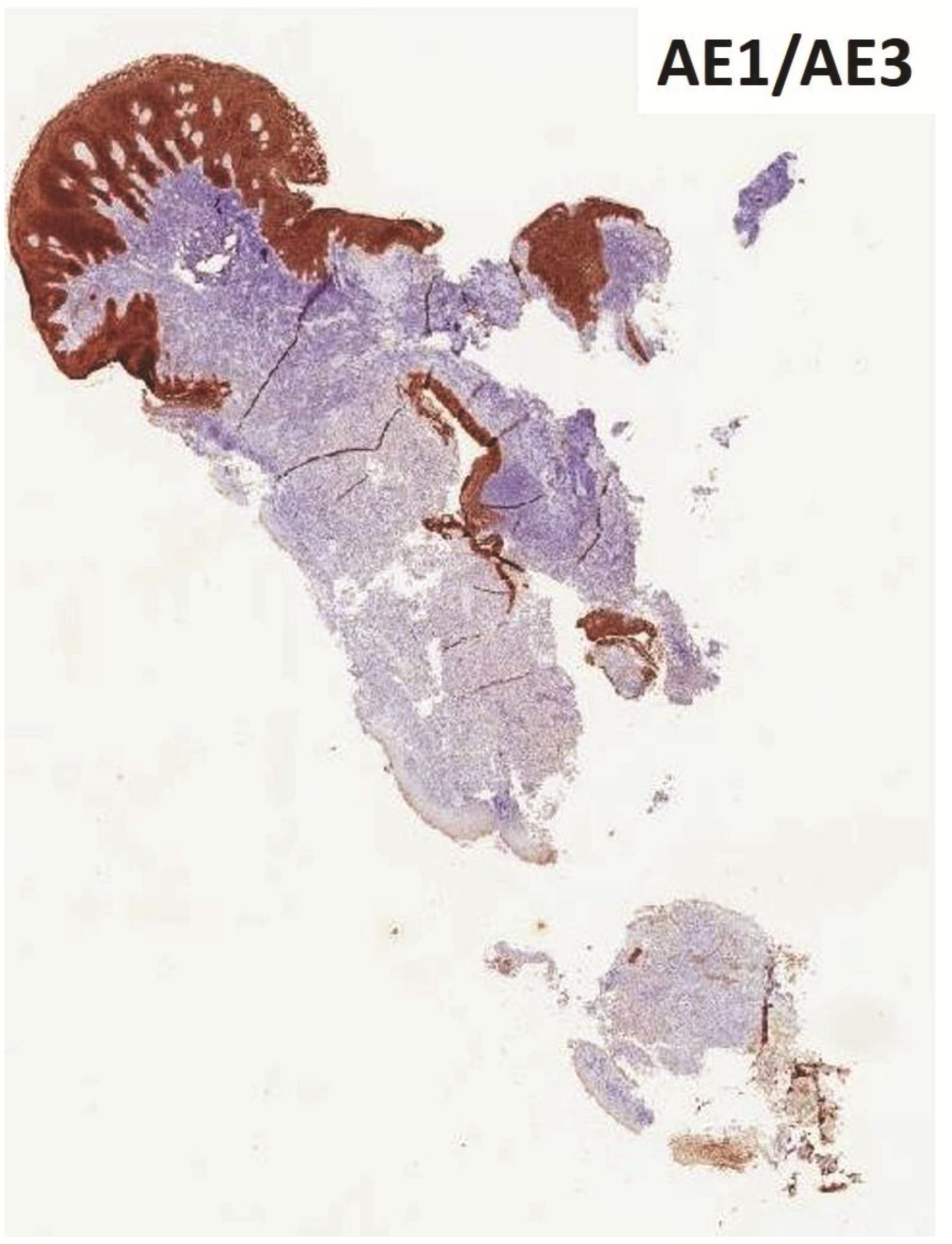
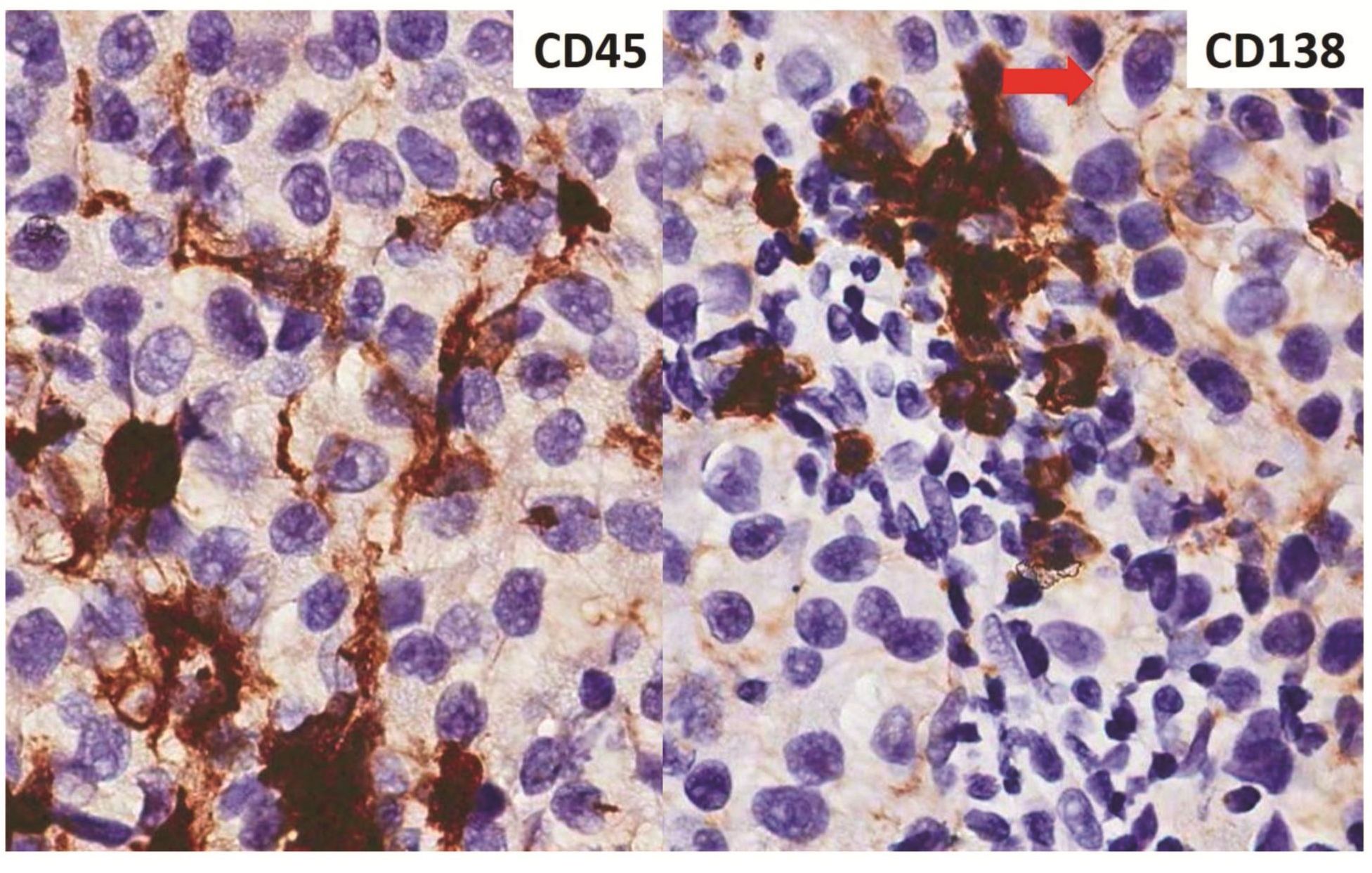
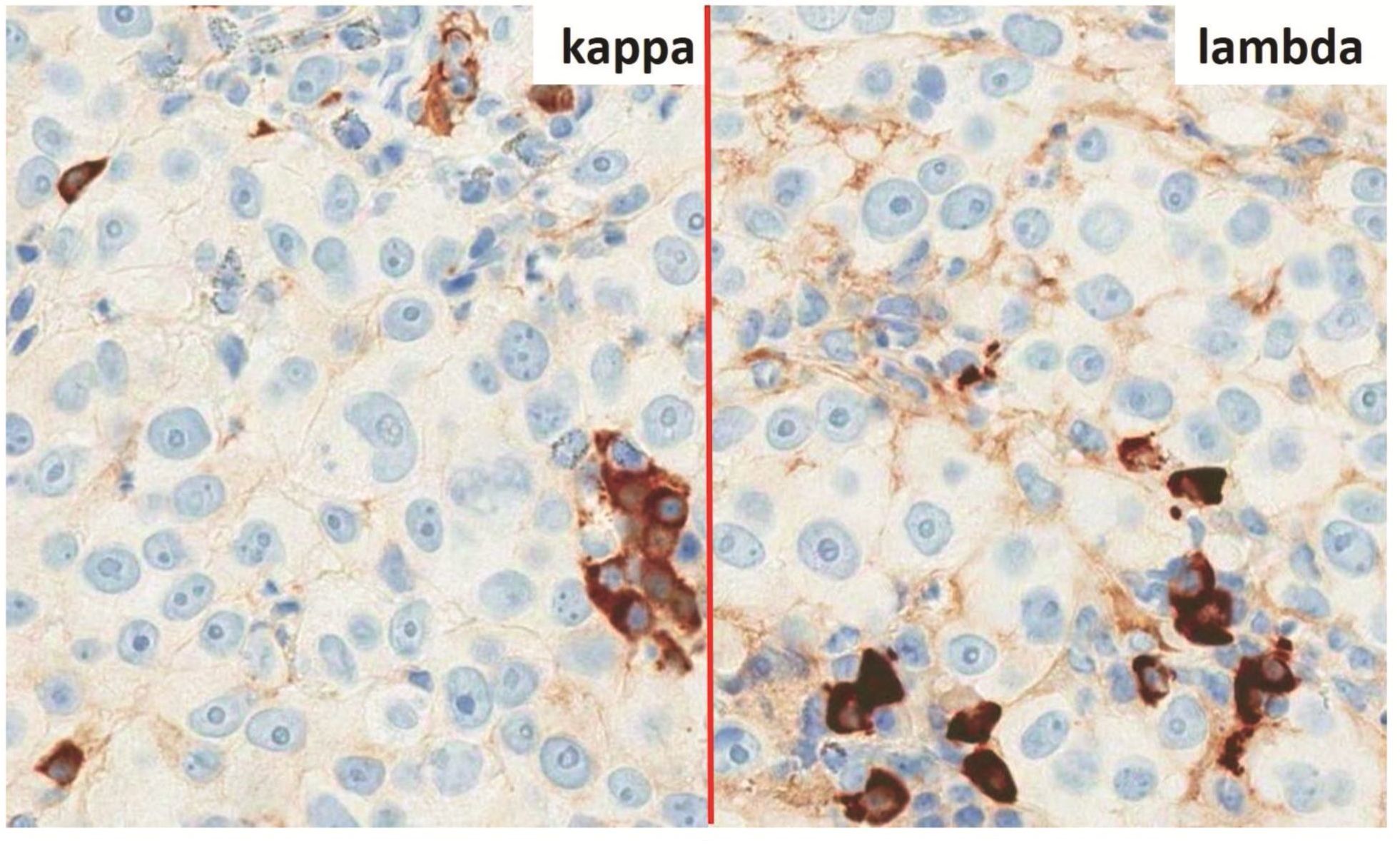
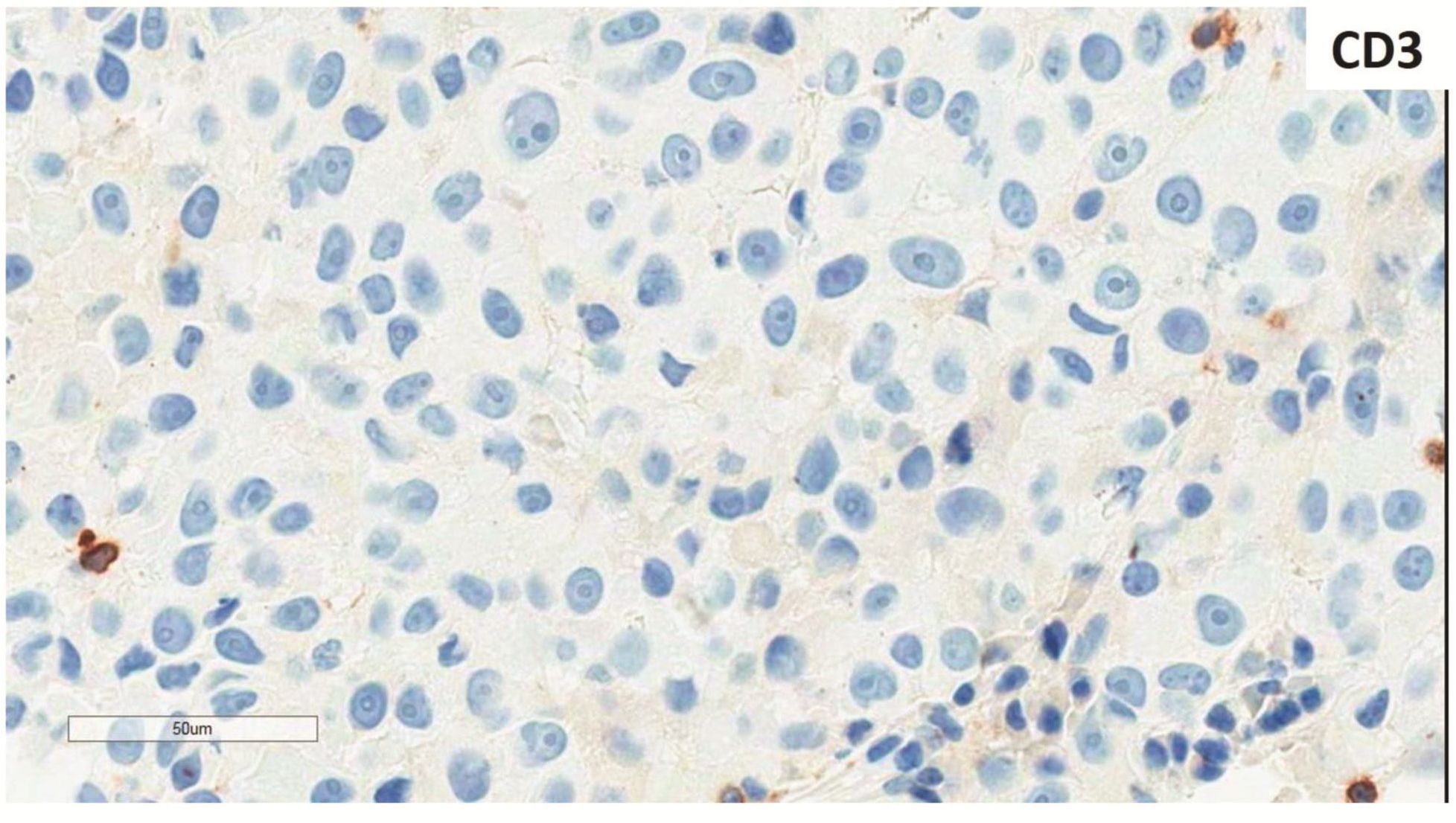
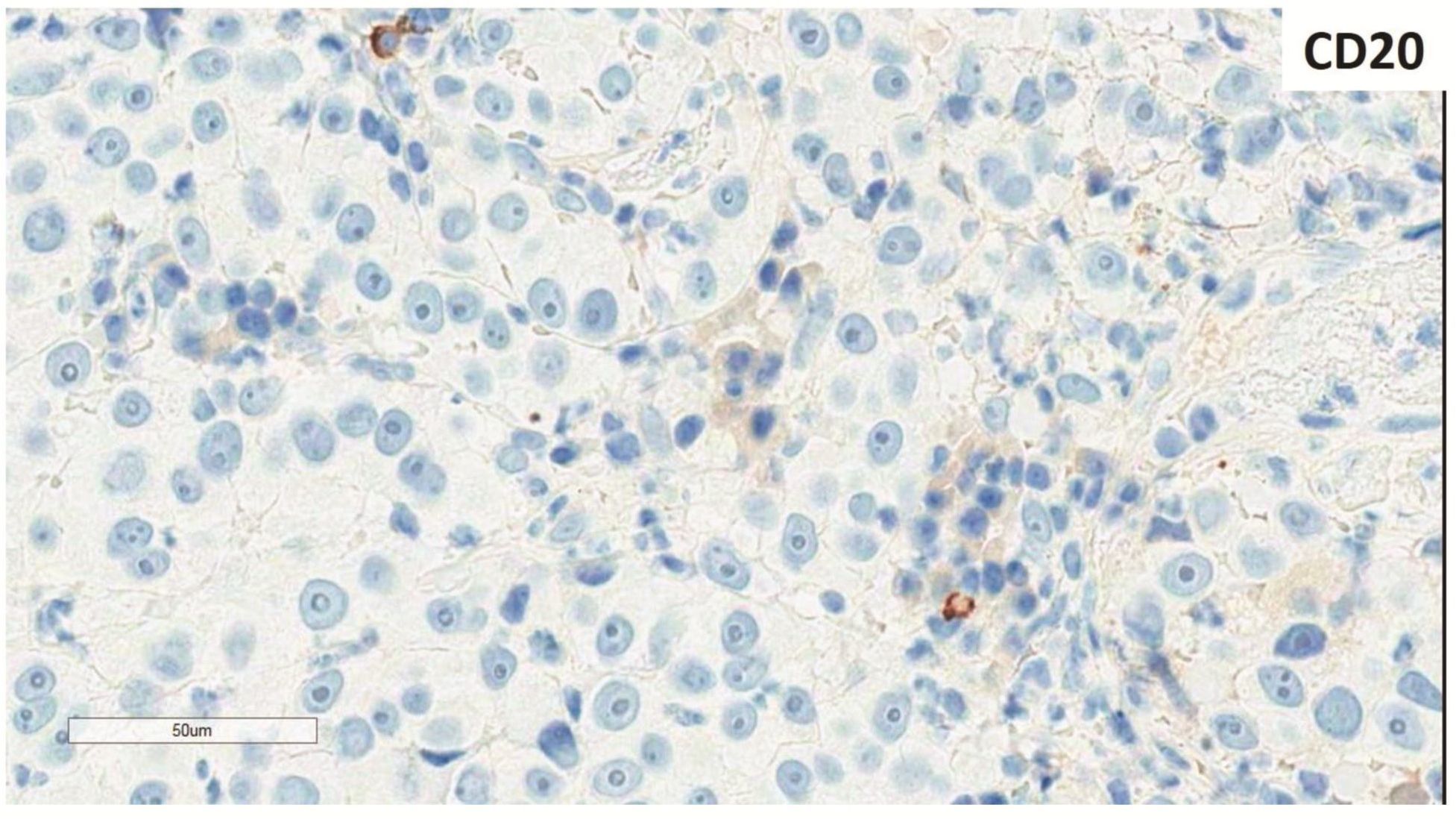
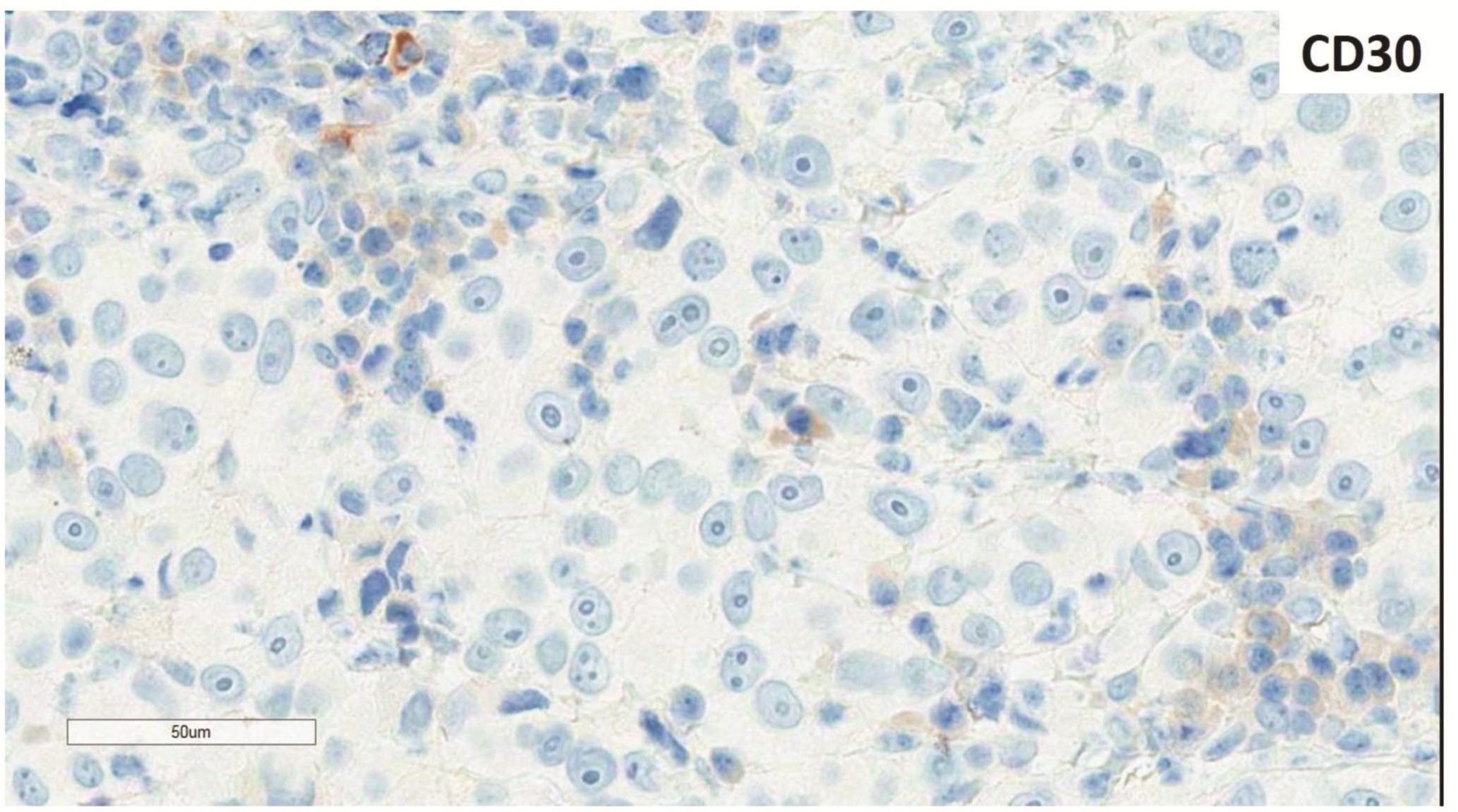
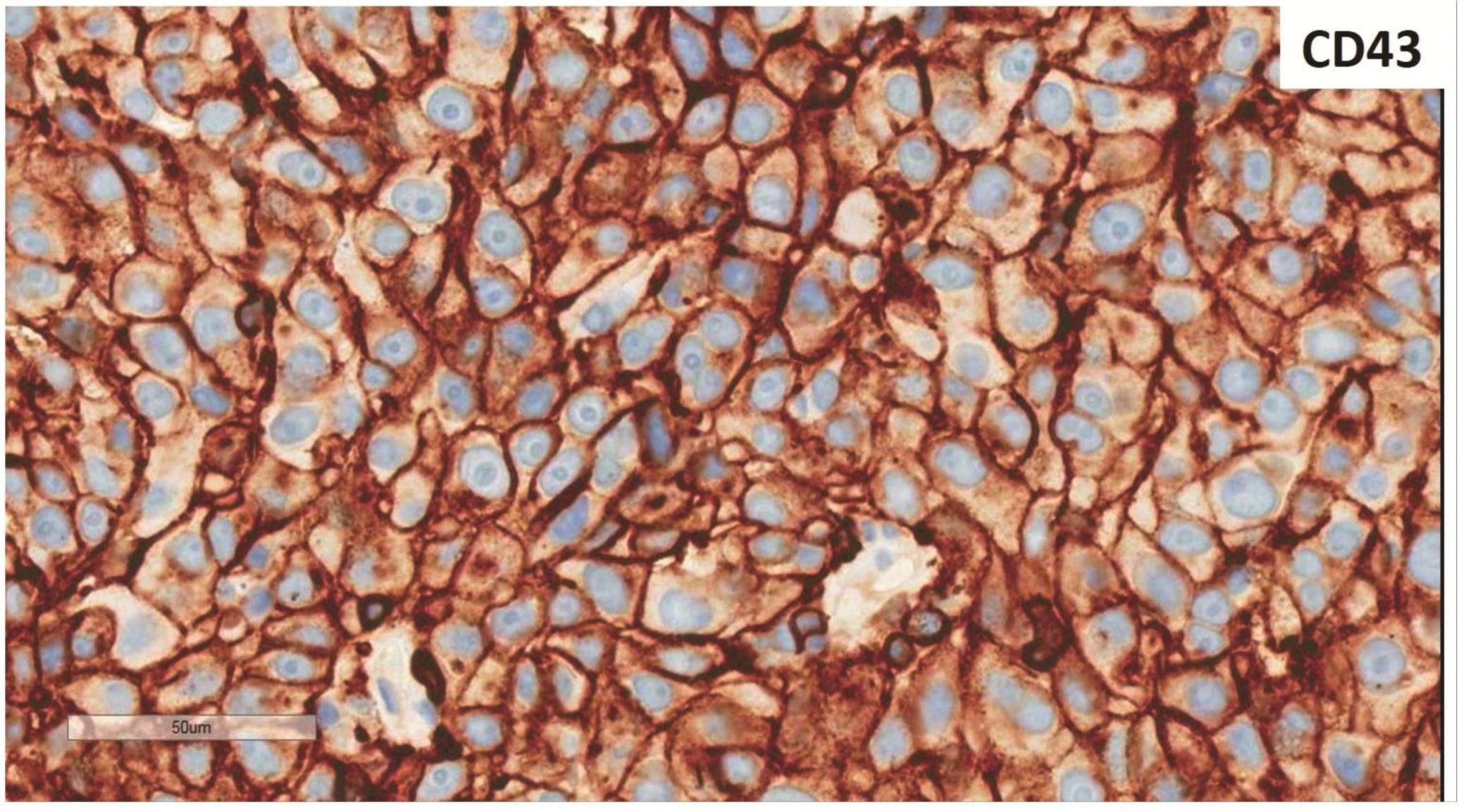
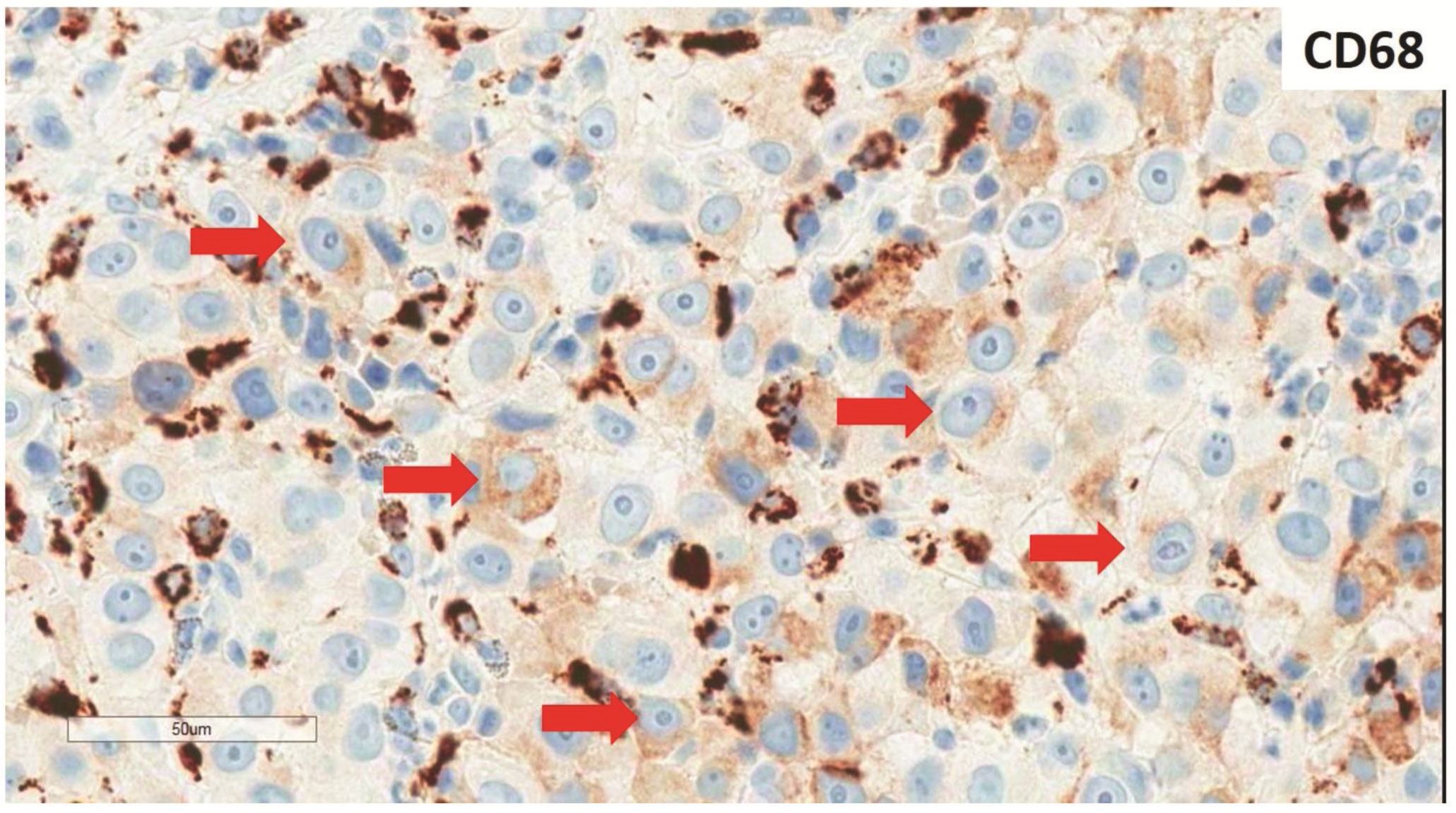
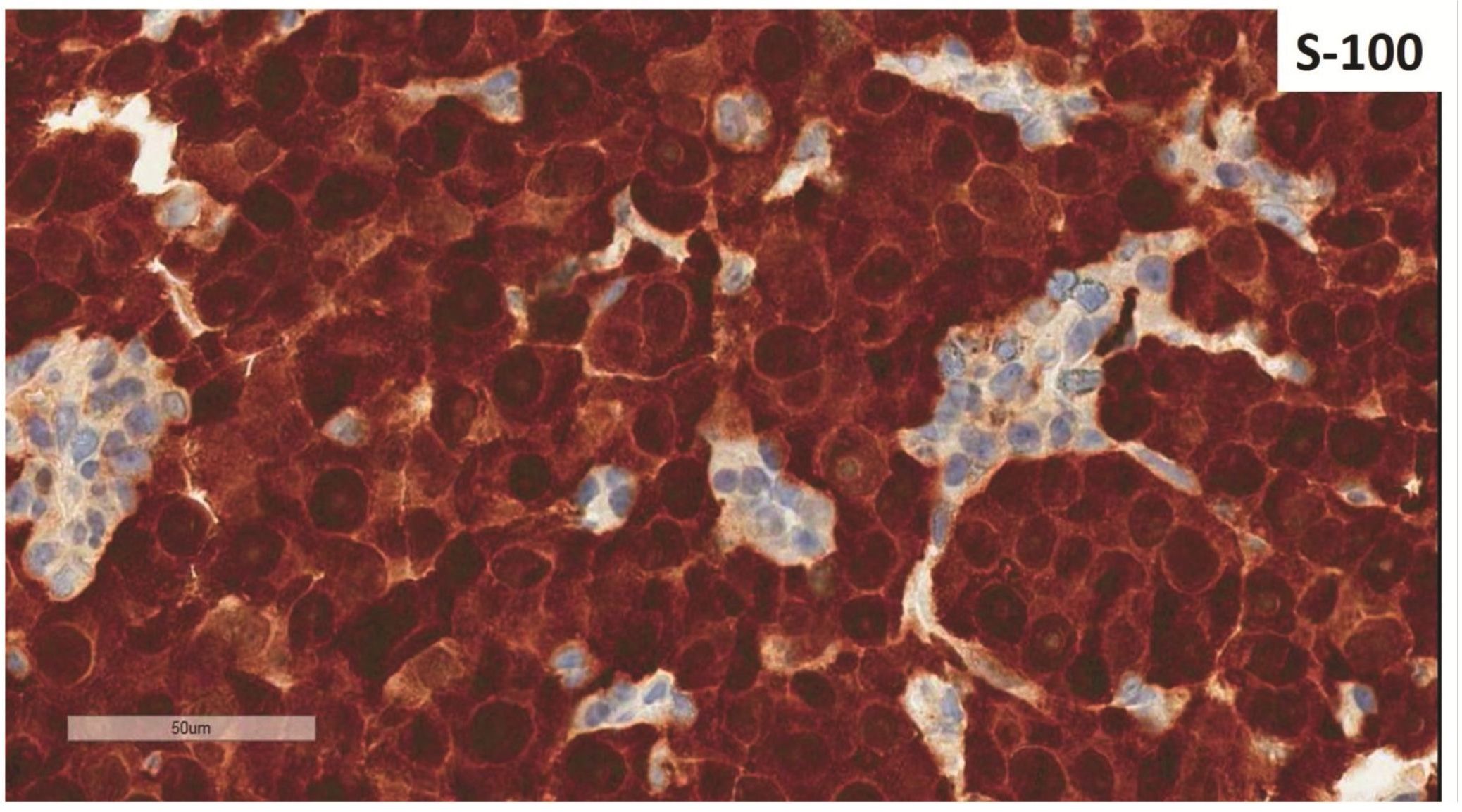
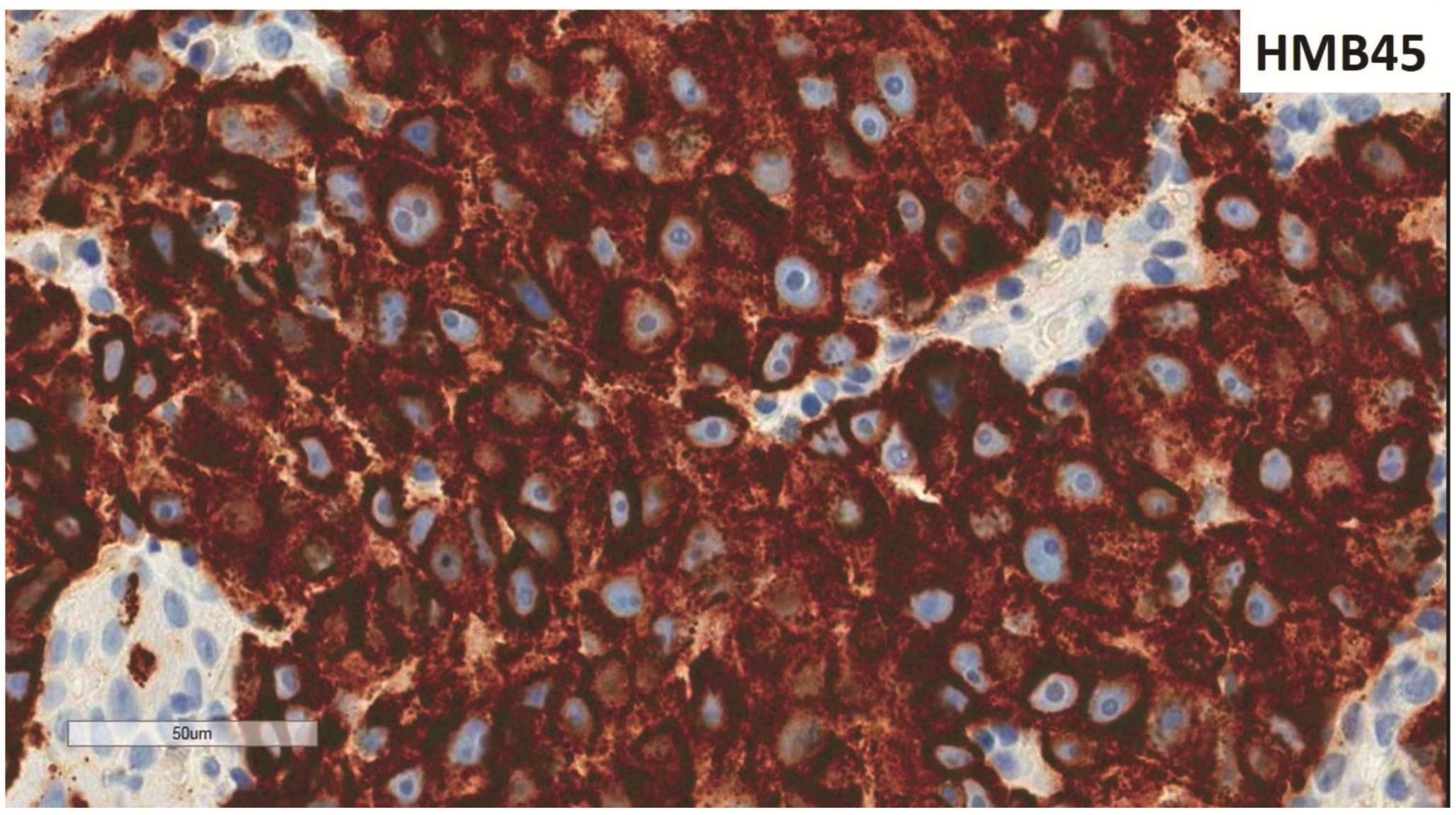
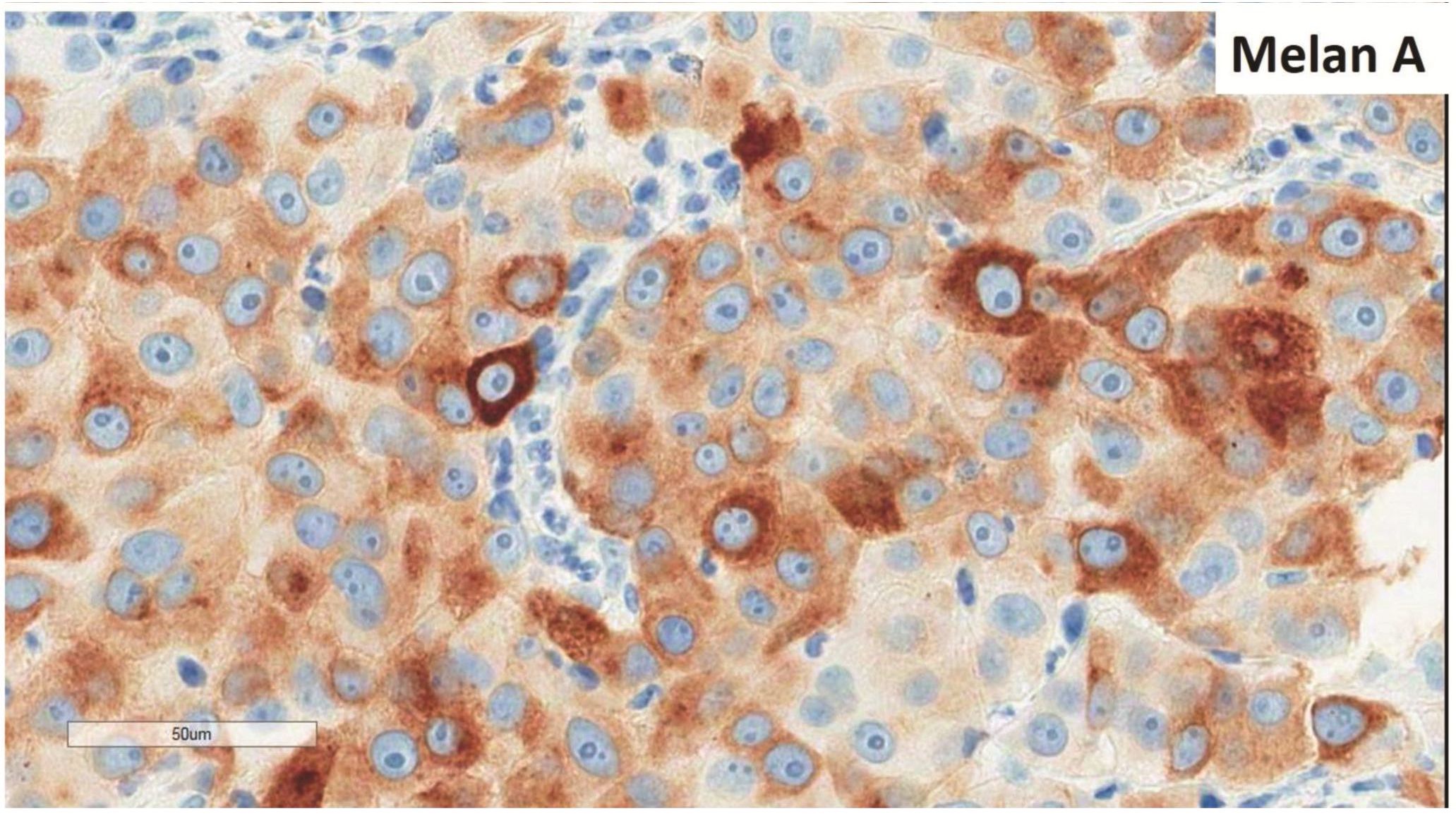
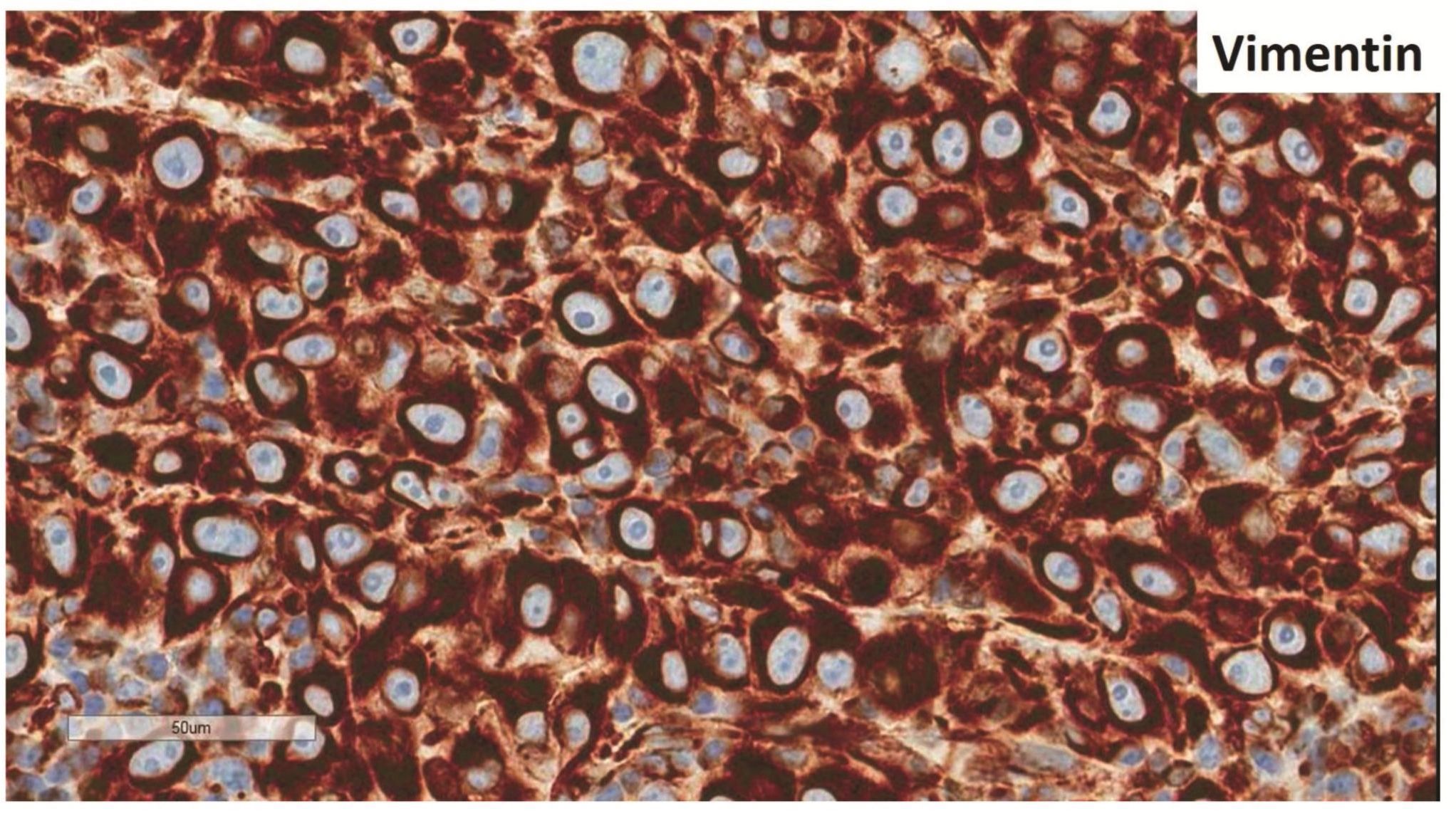
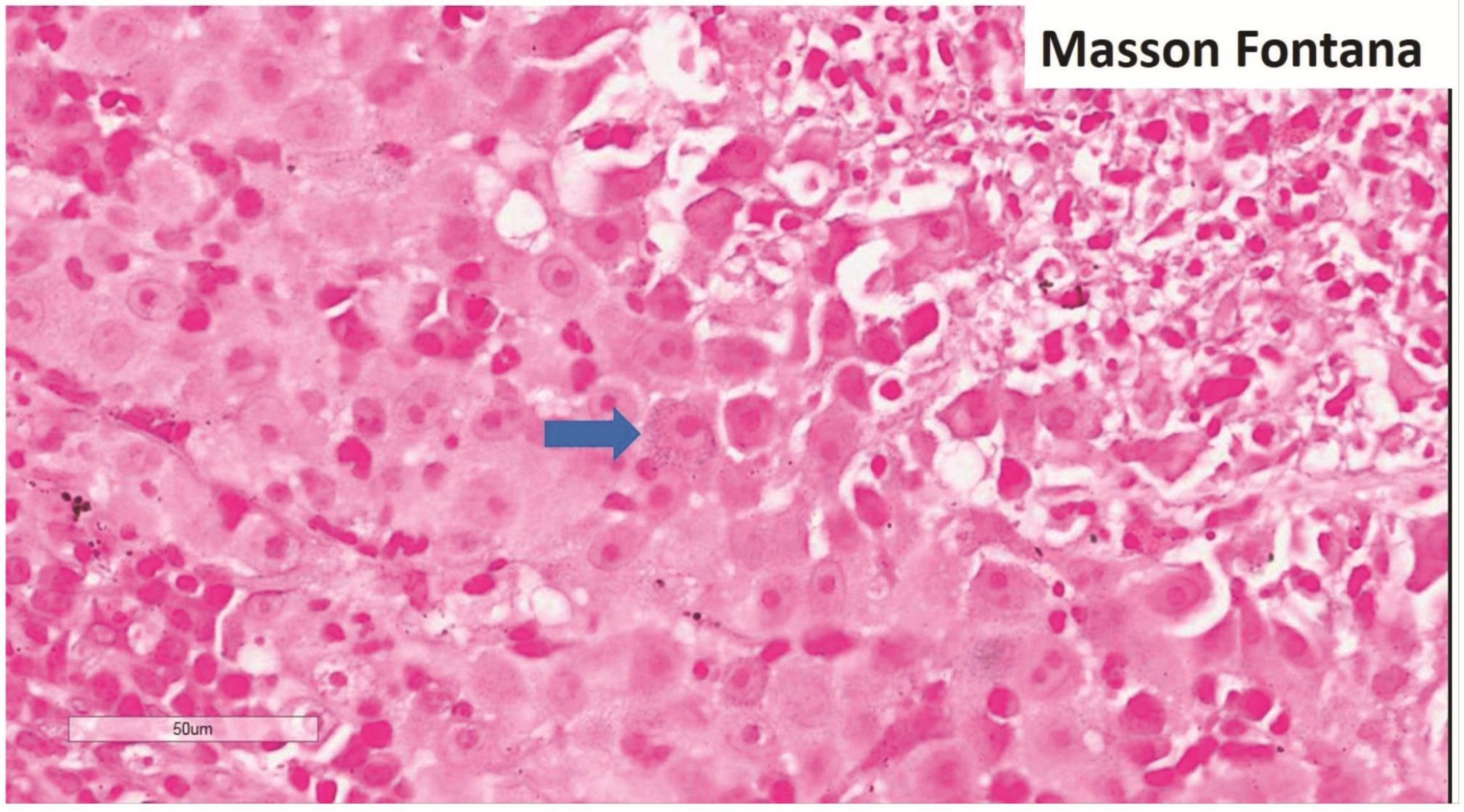
Morphologic evaluation showed ulceration of tonsillar surface epithelium by sheets of large tumor cells underneath. (Figure 1) The tumor cells had distinct central nucleolus and modest amount of pink cytoplasm. (Figure 2) The differential diagnosis included undifferentiated large cell carcinoma, hematologic malignancy, and malignant melanoma. The original immunostained slides showed focal and faint CD138 expression (membrane pattern) but negative AE1/AE3 keratins and CD45. The tumor cells expressed CD43, CD68 (focal), S-100 protein, HMB45, Melan A, and vimentin. The negative markers included CD3, CD20, CD30, CD79a, MUM1, PAX5, kappa, lambda, and CK5/6. The proliferation index was high (70-75%) by Ki-67. (Figure 3 A to N) Masson Fontana stain showed fine melanin pigment granules in only few tumor cells. (Figure 3O)
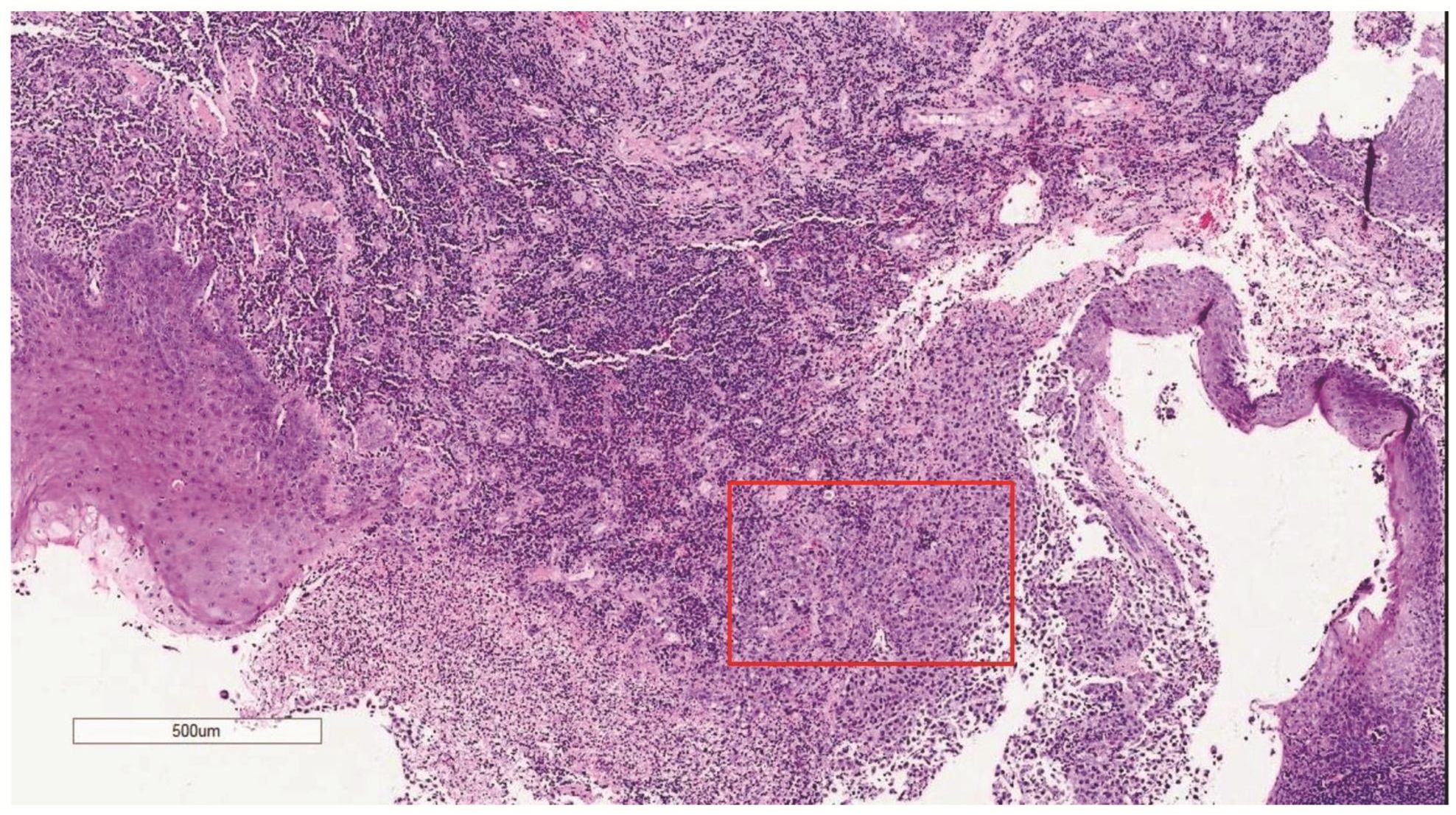
Figure 1 Destruction of the surface epithelium of the tonsil by large neoplastic cells (red box for higher magnification shown in Figure 2).
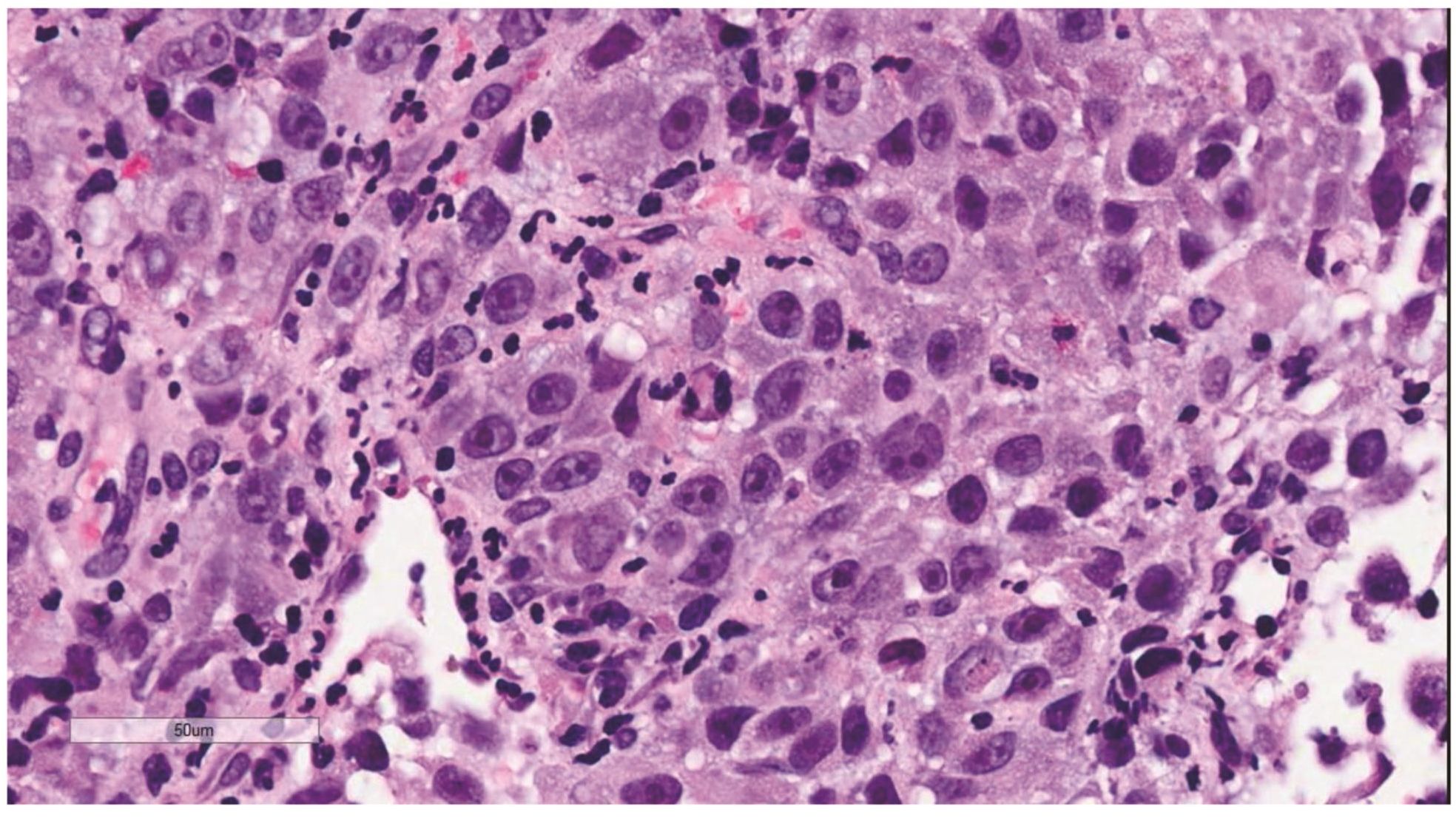
Figure 2 Large neoplastic cells with distinct central nucleolus and eosinophilic cytoplasm. They were interpreted as plasmablasts in the original pathology report.

Figures 3 Compilation of immunohistochemical findings and Masson Fontana stain.
A) AE1/AE3 keratins. Only positive for the intact surface epithelium but negative in the neoplasm underneath. B) CD45-negative. C) Few CD138-positive cells (red arrow). D & E) Negative for both kappa & lambda. Note occasional kappa+ or lambda+ plasma cells. F) CD3-negative. G) CD20-negative. H) CD30-negative. I) CD43-positive. Note distinct membrane staining and occasional paranuclear accentuation. J) CD68-positive in few neoplastic cells (red arrows). K) S-100 protein-positive. Note typical nuclear and cytoplasmic staining and clear negative reactive cells in the background. L) HMB-45-positive. Note distinct granular cytoplasmic staining. M) MelanA-positive. Note variable intensity of cytoplasmic staining. N) Vimentin-positive. Note intense cytoplasmic staining. O) Melanin pigments shown in a neoplastic cell (blue arrow) by Masson Fontana.
DISCUSSION
According to the results shown above, this is malignant melanoma that hardly contains melanin pigments or the so-called “amelanotic melanoma.” Certainly, this variant often produces diagnostic difficulty for pathologists6. As demonstrated in the present case, the original diagnosis was plasmab-lastic lymphoma based on the morphology of large tumor cells with distinct nucleolus and the misinterpretation of CD138 positivity while keratins and CD45 were negative. Based on the WHO classification (2008) for hematopoietic and lymphoid tissue, the diagnosis of plasmablastic lymphoma is based on morphology of immunoblasts with the phenotype of plasma cell7. However, CD138 alone does not mean plasma cell phenotype as it can express in many kinds of tumors8. Furthermore, review of the original immunostained slide for CD138 in the present case showed only few positive tumor cells. (Figure 3C) In practice, monoclonal plasma cell phenotype should be demonstrated for making a diagnosis of plasmablastic lymphoma to avoid the CD138 positivity in tumors of other than plasma cell phenotype. In the present case, the failure to demonstrate immunoglobulin light chain restriction led to further characterization of the tumor cells. Sometimes when the plasmacytic neoplasm fails to show immunoglobulin light chain restriction, one may try to evaluate restriction to immunoglobulin heavy chain before excluding plasmacytic neoplasm.
Practically, a “basic kit” to determine the phenotype of the tumor cells includes CD45 (leukocyte common antigen), keratins, and S-100 protein in case of undifferentiated or poorly differentiated neoplasm, no matter the size of tumor cells6. But, this basic kit has not been applied every time as it depends on the differential diagnosis after morphologic evaluation by an individual pathologist. For example, in the present case, the first pathologist included only carcinoma, lymphoma, and plasma-blastic lymphoma in the differential diagnosis as evidenced by a limited panel of immunostaining for keratins, CD45, and CD138. The diagnosis of plasmablastic lymphoma was made after CD 138+ tumor cells were found, even only few, while the other two markers were negative. Upon the review, after failure to show immunoglobulin light chain restriction, a broader panel of immunostaining was performed. Certainly, S-100 protein was included in the panel, then additional immunostaining for other markers of malignant melanoma such as HMB-45 and Melan A. If malignant melanoma was not included in the differential diagnosis, the present case would have been diagnosed as peripheral T-cell lymphoma, not otherwise specified type based on the CD43+ CD30-phenotype. Fortunately, this pitfall did not occur in the present case upon the review.
CD43 has been characterized as sialophorin, leukosialin, leukocyte sialoglycoprotein. This molecule is a surface antigen that is defective in Wiskott-Aldrich syndrome and may participate in activation of T-cells. Several clones of antibodies have been raised against the antigenic epitopes of the CD43 molecule such as MT1, DF-T1, L60 (Leu-22), L10, BS1, YG5, 2C8, and 8E101-3, 9; the antibody from the L60 clone was used in the immunostaining of the present case. CD43 is mainly expressed on cell surface of T-cells and various types of marrow hematopoietic cells except for mature erythrocytes and B-cell subsets. In tumor cells, CD43 has been used as a preferential T-cell marker but it is now known to express in many kinds of tumor cells, including some small B-cell lymphoid neoplasms such as small lymphocytic lymphoma/chronic lymphocytic leukemia and marginal zone lymphoma, myeloid malignancy, plasmacytic neoplasm, and various types of carcinomas from different primary sites such as urinary bladder, kidney, prostate, testis, breast, lung, stomach, thyroid, larynx, etc. Recently, altered glycosyla-tion in some cancers can be detected by a particular monoclonal antibody raised to recognize cancer-associated CD43 glycoforms. So CD43 is one of many glycan biomarkers discovered by glycomics and gly-coproteomics with the hope of better sensitivity and specificity for early cancer detection, evaluation of cancer treatment, and assessment of prognosis3.
In summary, the present case report has demonstrated a diagnostic pitfall caused by a limited panel of immunostaining and misinterpretation of the positive immunostaining result. Once again, the “basic kit” of immunostaining for CD45, keratins, and S-100 protein should be performed at the first step of characterization of undifferentiated or poorly differentiated tumor. A panel for keratins, vimen-tin, S-100, CD45, CD30, and CD138 will be helpful when dealing with undifferentiated large cell tumor. Finally, CD43, once commonly used as a preferential T-cell marker, should be used with caution as already demonstrated by the present case report.
CONFLICT OF INTEREST
The author declares that he has no conflict of interest.
ACKNOWLEDGMENTS
The author would like to thank all personnel at Department of Pathology, Faculty of Medicine Siriraj Hospital, Mahidol University for their support.
REFERENCES
1. Stross WP, Warnke RA, Flavell DJ, et al. Molecule detected in formalin fixed tissue by antibodies MT1, DF-T1, and L60 (Leu-22) corresponds to CD43 antigen. J Clin Pathol 1989;42:953-61.
2. Santamaria M, Lopez-Beltran, Mariano T, Pena J, Molina IJ. Specific monoclonal antibodies against leukocyte-restricted cell surface molecule CD43 react with nonhematopoietic tumor cells. Cancer Res 1996;56:3526-9.
3. Tuccillo FM, de Laurentiis A, Palmieri C, et al. Aberrant glycosylation as biomarker for cancer: Focus on CD43. BioMed Research International 2014, Article ID 742831,13 pages http://dx.doi. org/10.1155/2014/742831.
4. Buehler D, Waknitz M, Rehrauer W, Ranheim E, Selvaggi S. Small cell variant of malignant melanoma masquerading as lymphoma on fine-needle aspiration cytology: A case report. Diagn Cytopathol 2012;40:619-23.
5. Mikel UV (ed). Armed Forces Institute of Pathology. Advanced Laboratory Methods in Histology and Pathology. Washington, DC: American Registry of Pathology, 1994.
6. Rosai J. Rosai and Ackerman’s Surgical Pathology, 10th ed. China: Mosby, 2011.
7. Swerdlow SH, Campo E, Harris NL, et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon: IARC, 2008.
8. Kambham N, Kong C, Longacre TA, Natkunam Y. Utility of syndecan-1 (CD138) expression in the diagnosis of undifferentiated malignant neoplasms: a tissue microarray study of 1,754 cases. Appl Immunohistochem Mol Morphol 2005;13:304-10.
9. Kim S, Hong JW, Cho WD, et al. Characterization of Two Novel mAbs Recognizing Different Epitopes on CD43. Immune Netw 2014; 14:16470.


