Matrix producing clear cell primary pulmonary myoepithelial carcinoma: a case report with a review of the literature
Gittwa Vatsaraj Kottangal*, Shalini Kuruvila and
Kavitha Kanjirakkattumana Parameswaran
Department of Pathology, Aster MIMS, Kozhikode, Kerala, India
Correspondence to: Dr Gittwa Vatsaraj Kottangal, Department of Pathology, Aster MIMS, Kozhikode, Kerala 673016 India. Telephone: 009 170 259 552 93 Email: drgitwa@hotmail.com
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
|
Submitted: |
11 April 2021 |
|
Accepted: |
21 April 2021 |
|
Published: |
1 January 2021 |
Abstract
Myoepithelial carcinoma, also known as malignant myoepithelioma, is mainly encountered in the salivary glands and, at a lower incidence, in the sweat glands (skin), breast, soft tissue, bone and very rarely in the lung. Pulmonary myoepithelial carcinoma was first described by Higashiyama et al. in 1998, and to the best of our knowledge, only 15 cases have been reported in the English literature so far. The reports are that of 13 adults and 2 paediatric cases. Herein we report a case of a matrix producing clear cell primary pulmonary myoepithelial carcinoma in a 64-year-old male patient who presented with chronic cough and respiratory distress. The characteristic histomorphological and the immunohistochemical features of the tumour were used to further discuss the distinct clinicopathologic features, differential diagnosis, treatment and prognosis of the entity.
Keywords: histomorphology; immunohistochemistry; matrix; myoepithelial; pulmonary
Introduction
Myoepithelial carcinoma of the lung is a rare neoplasm, accounting for 0.1 – 0.2% of all lung tumours. This tumour arises from the submucosal bronchial glands, which are considered to be minor salivary glands. The various benign and malignant salivary gland type tumours of the lung include mucoepidermoid carcinoma, adenoid cystic carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, acinic cell carcinoma, oncocytoma, myoepithelioma and myoepithelial carcinoma. Considering the lack of ductal/glandular differentiation, 2015 World Health Organization (WHO) classification of lung tumours enclosed myoepithelial carcinoma as a new separate entity differentiating it from epithelial-myoepithelial carcinoma and pleomorphic adenoma. Ultrastructurally, myoepithelial cells show both epithelial and smooth muscle differentiation features, with myofilaments, tonofilaments, desmosomes and external lamina. This differentiation is the reason for the varying histological morphology of the myoepithelial tumours. This report describes the case of a 64-year-old male patient with primary myoepithelial carcinoma of the lung with clear cell morphology.
Case Report
A 64-year-old non-smoking man presented in the Department of Pulmonology with non-productive chronic cough and dyspnoea of 4 months duration. A chest X-ray revealed mass lesion. Positron emission tomography – computed tomography (PET-CT) showed abnormally increased tracer uptake in enhancing peripheral areas of a thick-walled cavitary lesion (measuring 6.0 x 5.4 x 5.7 cm) in the superior segment of the lower lobe of the left lung, extending to hilum (Figure 1). Additionally, there was a nodular soft tissue density lesion (measuring 2.8 x 2.4 cm) with lobulated margins in the anterior segment of the upper lobe of the left lung (Figure 2) and metabolically active discrete mediastinal lymph nodes, i.e. left lower paratracheal, left hilar, subaortic and left peribronchial lymph nodes (Figure 1).
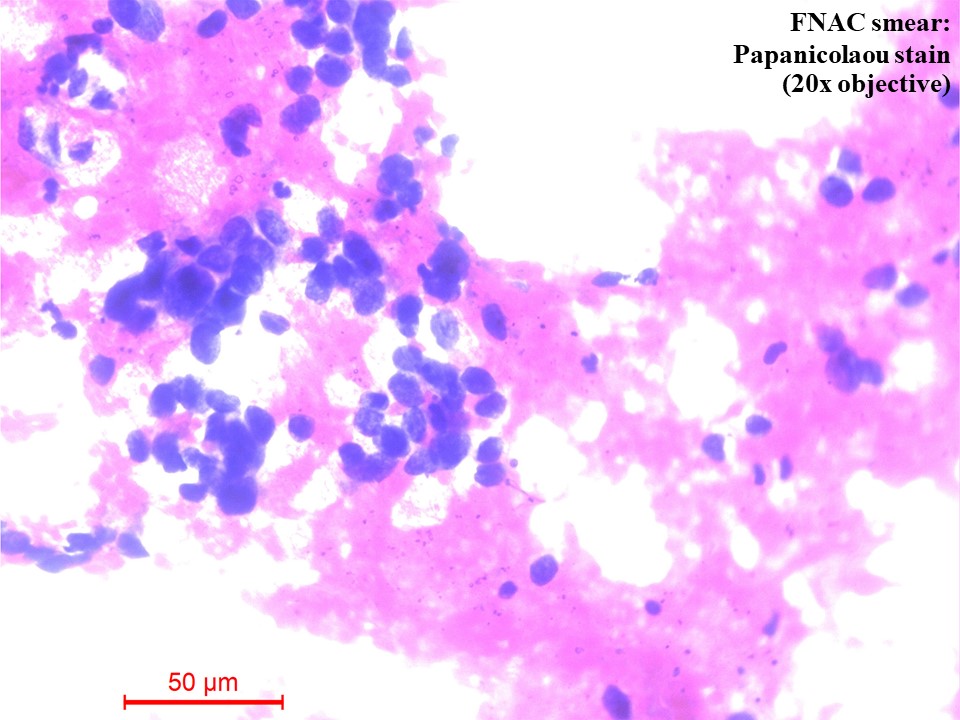
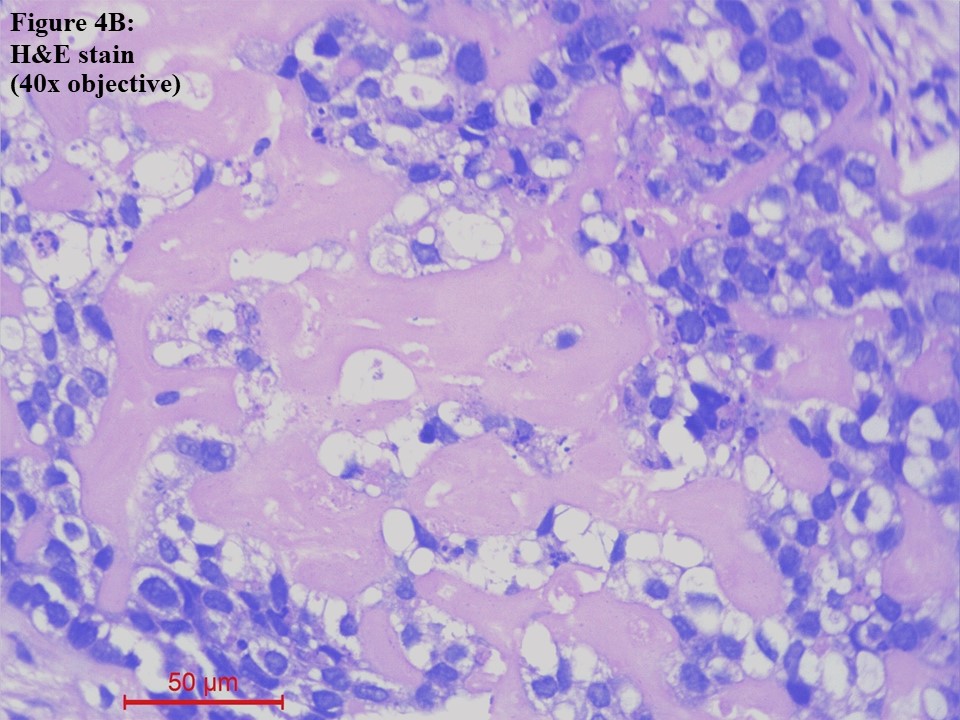
Computed tomography (CT) – guided fine needle aspiration cytology (FNAC) and Tru-Cut biopsy were taken from the soft tissue density lesion in the anterior segment of the left lung upper lobe. FNAC smear was of low cellularity, showing poorly cohesive clusters of malignant cells with mildly pleomorphic hyperchromatic nuclei and a moderate amount of pale eosinophilic to clear cytoplasm. The background showed pale eosinophilic material (Figure 3). Tru-Cut biopsy showed lung tissue infiltrated by a neoplasm composed of cells with hyperchromatic nuclei and a moderate amount of pale eosinophilic to clear cytoplasm arranged as cords, nests and fused glands in blobs of an acellular pale eosinophilic matrix. Comedo necrosis and 12 – 13 mitoses per 10 high-power fields (HPFs) were seen (Figures 4A and 4B).
Figure 1 Positron emission tomography – computed tomography (PET-CT) of the chest. There was abnormally increased tracer uptake in enhancing peripheral areas of a thick-walled cavitary lesion in the superior segment of the lower lobe of the left lung, extending to the hilum (white arrow) and metabolically active discrete mediastinal lymph nodes (blue circle).
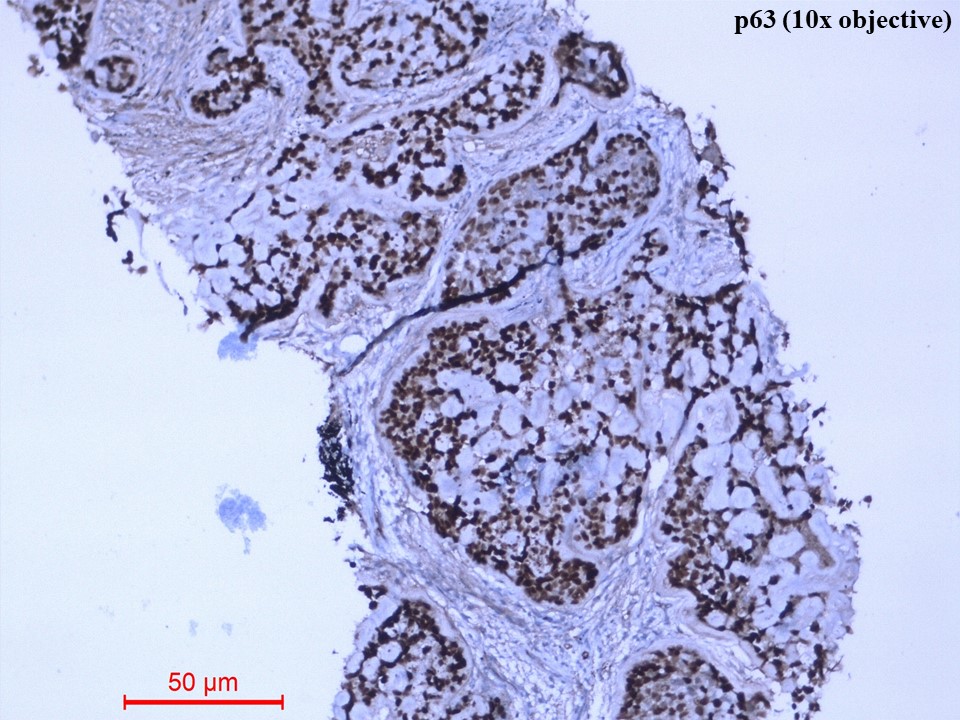
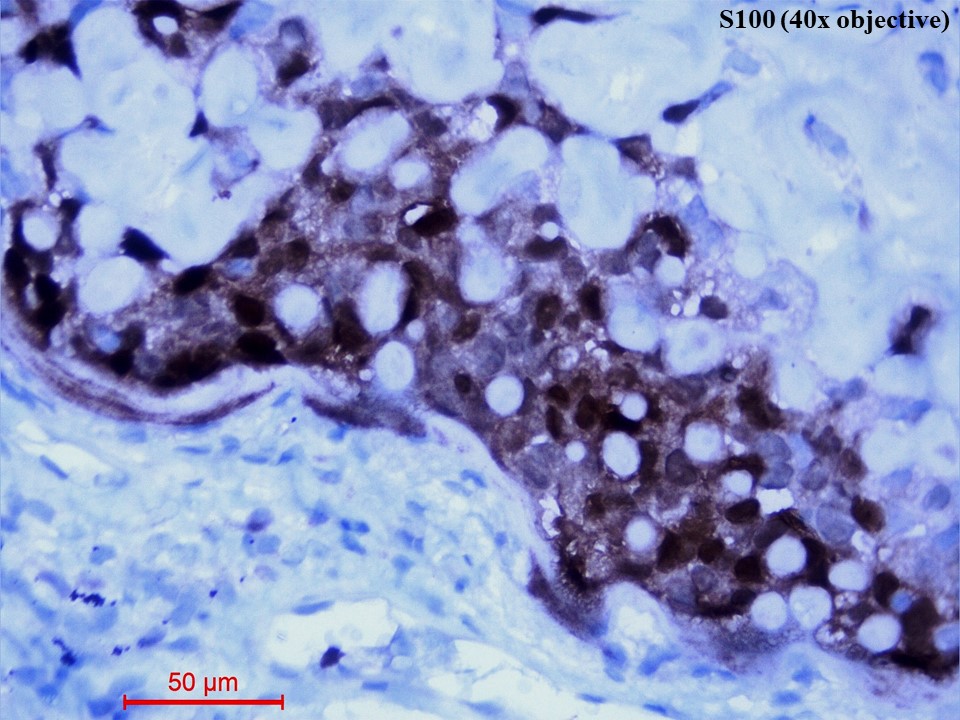
Immunohistochemistry showed diffusely strong positivity for p63 (Figure 5) and moderate positivity for S100 (Figure 6). There was negative immunoexpression for CD10, CD117, CK7, HMB-45, synaptophysin and TTF1. With the above histomorphological and immunohistochemical features, the diagnosis of primary myoepithelial carcinoma of the lung, clear cell type was given. Due to poor health conditions, surgery was not done, and the patient was put on chemotherapy. For 9 months, the patient shows no progression of the disease and is alive with the disease.
Figure 2 Positron emission tomography – computed tomography (PET-CT) of the chest. There was nodular soft tissue density lesion with lobulated margins in the anterior segment of the upper lobe of the left lung (blue circle).
Figure 3 Fine needle aspiration cytology (FNAC) smear of the lung mass [Papanicolaou (PAP) Stain, 20x objective]. There were poorly cohesive clusters of malignant cells with mildly pleomorphic hyperchromatic nuclei and a moderate amount of pale eosinophilic to clear cytoplasm. The background showed pale eosinophilic material.
(A). 10x objective
(B). 40x objective
Figure 4 Lung tissue obtained from Tru-Cut biopsy [haematoxylin and eosin (H&E) stain]. (A). The lung tissue was infiltrated by a neoplasm arranged as cords, nests and fused glands in blobs of an acellular pale eosinophilic matrix (10x objective). (B). The cells had moderately pleomorphic hyperchromatic nuclei and a moderate amount of pale eosinophilic to clear cytoplasm (40x objective).
Figure 5 Immunohistochemistry of p63 protein (10x objective). The tumour cells showed diffusely strong nuclear immunostaining of p63.
Figure 6 Immunohistochemistry of S100 protein (40x objective). The tumour cells showed both nuclear and cytoplasmic immunostaining of S100.
Discussion
In 1943, Sheldon identified myoepithelial tumours as a distinct entity in salivary glands(1), while the malignant counterpart - myoepithelial carcinoma - was first described by Stromeyer et al. in salivary glands in 1975(2). In 1987, Strickler et al. reported the first case of a benign myoepithelioma in the lung(3). While primary pulmonary myoepithelial carcinoma is first described by Higashiyama et al. in 1998(4). Myoepithelial tumours were recognised as a histologically distinct entity by the WHO in 1991 in salivary gland tumours(5). While myoepithelial tumours/carcinomas entered as a separate entity in the 2015 WHO classification of lung tumours(6). Primary salivary gland – type lung tumours are very rare and represent less than 1% of all lung tumours(7,8). Pulmonary primary myoepithelial carcinoma is even rare, encountering only 0.1 – 0.2% of all lung tumours.
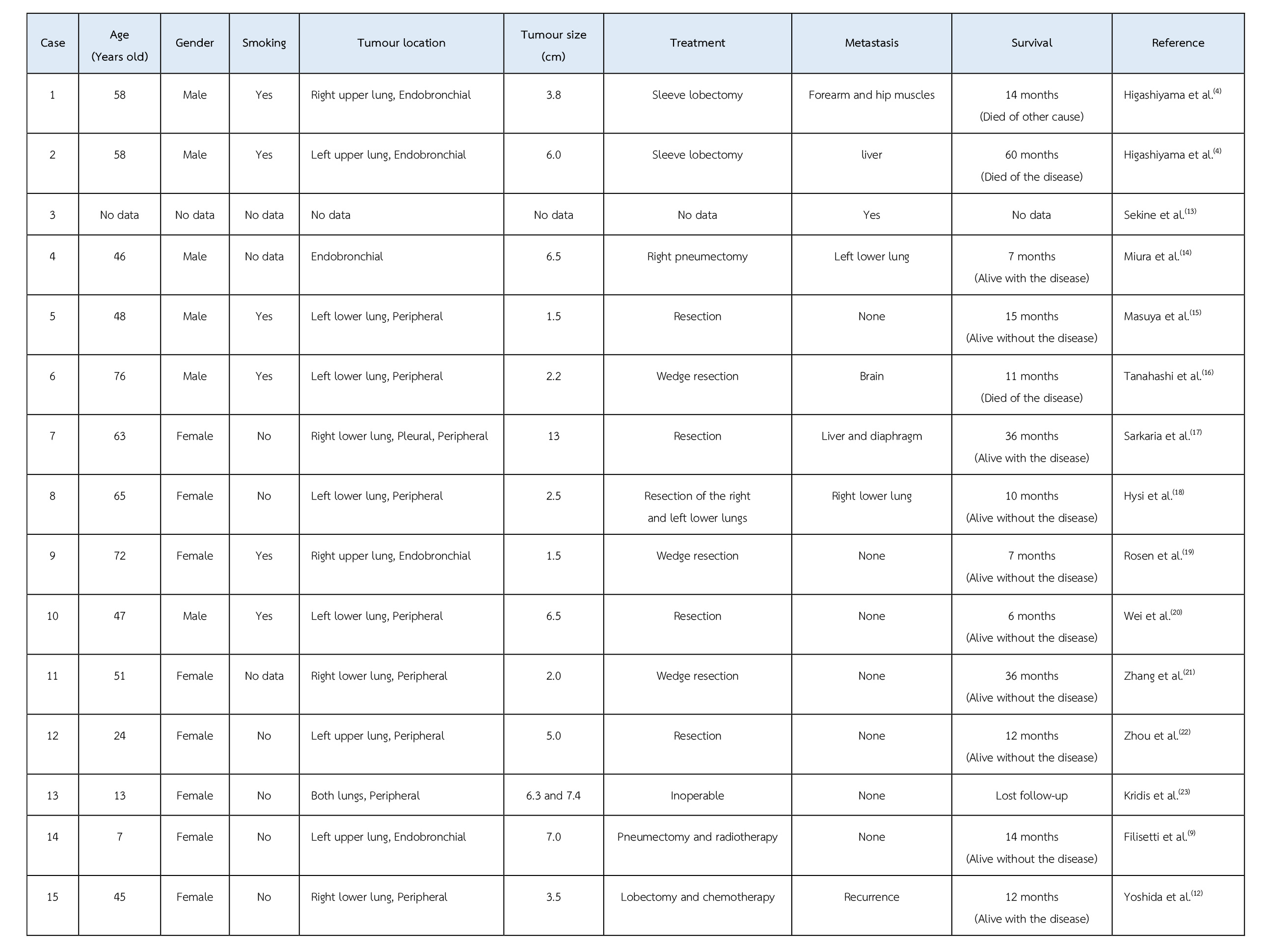
To the best of our knowledge, only 15 cases of primary pulmonary myoepithelial carcinoma have been reported in the English literature(9). The clinical data of these reported cases are shown in the Table. Adult cases range in age from 24 to 76 years old (mean age of 55.5 years) and paediatric cases are that of 7- and 13-year-old girls. The male-to-female ratio is 7:8. Six out of 16 cases have a history of smoking. Five cases presented as endobronchial mass and 11 cases had peripheral mass. Except 2 cases, all underwent resection. Six cases had metastasis. One case had a recurrence. Seven cases were alive without disease. Five cases were alive with disease. Two cases died of the disease. One case died due to other cause. However, two cases had unavailable follow-up data.
Myoepithelial cells are seen in the secretory organs like salivary glands, breast, sweat gland, prostate, etc. These cells surround acini and intercalated ducts and express a dual epithelial and smooth muscle phenotype. They are thought to be ectodermal in origin(1). The neoplastic behaviour of these cells is at times unpredictable and forms either a major component of the myoepithelial entities (like myoepithelioma and myoepithelial carcinoma) or as one of the components of the tumours like epithelial, myoepithelial carcinoma, pleomorphic adenoma and adenoid cystic carcinomas(4).
Table The clinical data of fifteen reported cases of primary pulmonary myoepithelial carcinoma.
Myoepithelial carcinoma in the lung presents as either an endobronchial mass or a peripheral mass. The tumour develops from both the central and the peripheral bronchial glands. Grossly, the tumour is usually well-circumscribed, ranging from 1.5 to 20 cm, with a yellow-tan, sometimes glistering cut surface(6,10). Myoepithelial carcinoma is a malignant tumour that is entirely composed of myoepithelial cells with variable cellular morphologies, including spindle (most common), epithelioid, plasmacytoid, or clear cells (least common) along with a variable stromal component. It can be differentiated from myoepithelioma by the following criteria, i.e nuclear atypia, multinucleation, prominent nucleoli, high mitotic rate (an average of 13 mitoses per 10 HPFs) and necrosis. The tumour cells express epithelial markers (EMA and pan-CK) and at least one myoepithelial marker [caldesmon, calponin, glial fibrillary acid protein (GFAP), p40/p63, S100 and smooth muscle actin (SMA)](10). They will be negative immunostainings for CK7 (showing the absence of epithelial/glandular differentiation) and TTF1(6).
Frequent EWSR1 and FUS rearrangements(11) and case reports with SMARCB1 loss(12) are also seen. It is seen that specific histomorphological findings can be related to specific gene fusion products. Tumours with EWSR-PBX1 fusion were characterised by spindle cell proliferation with clear cytoplasm, while EWSR-ZNF444 translocation is seen in the epithelioid type of tumour cells. In a few cases with clear cell morphology, no specific fusion partners for EWSR were detected(11). SMARCB1 loss is found in the case with rhabdoid morphology(12).
Treatment for myoepithelial carcinoma is surgical resection. Unfavourable cases are also treated with chemotherapy and radiotherapy(12). Since the reported cases on this entity are very few, the knowledge regarding treatment is also limited. This tumour can metastasise to brain, contralateral lung, liver and soft tissue. A lower mitotic count is a favourable prognostic factor of clinical outcome and survival in primary myoepithelial carcinoma of the lung(6).
The differential diagnosis for the lung tumours with clear cell morphology includes PECOMA (clear cell/sugar tumour), hyalinising clear cell carcinoma, clear cell adenocarcinoma, clear cell variant of squamous cell carcinoma, epithelial myoepithelial carcinoma and metastatic clear cell carcinoma.
1. PECOMA (clear cell/sugar tumour)
This tumour consists of round or oval clear cells with abundant glycogen containing cytoplasm, which shows strong diastase-sensitive PAS positivity. Nucleoli may be prominent. Stroma is scanty with prominent thin-walled sinusoidal vessels. Mitosis and necrosis are extremely rare. Malignancy should be suspected if the tumour is infiltrative or shows significant mitotic activity. Immunohistochemistry shows positive for HMB-45 and S100. There is negative immunoexpression for pan-cytokeratin cocktail AE1/AE3, CK7, CK20 and p63/p40(24).
2. Hyalinising clear cell carcinoma
These tumours are of salivary gland origin. The tumour cells form sheets, nests, and cords. No definite glandular formation is seen. Stroma has thick parallel strands of fibrous tissue associated with the hyalinised and myxoid substance. Cells are polygonal to round with abundant pale eosinophilic to clear cytoplasm, bland nuclei with fine chromatin, small or inconspicuous nucleoli(25). Some cells show intranuclear pseudoinclusions, usually no necrosis, nuclear pleomorphism, or increase in mitosis. The periphery of the tumour shows chronic inflammatory infiltrate. Immunohistochemistry shows positivity for pan-keratin, CK7 and p63. Cells are negative for CD10, CK20, chromogranin, synaptophysin, HMB-45, napsin A, PAX-8 and TTF-1, and consistently negative for the myoepithelial markers like S-100, SMA and calponin. Molecular testing shows EWSR1-ATF1 fusion, which is also seen in clear cell sarcoma of soft tissue and angiomatoid fibrous histiocytoma(26).
3. Clear cell adenocarcinoma
The tumour shows predominantly clear cells arranged in glandular and papillary patterns. The cells are columnar and cuboidal with a moderate amount of clear cytoplasm, nuclear stratification seen with hyperchromatic nuclei, and small prominent nucleoli. Mitosis is seen. Special stains show no glycogen or mucins in the cytoplasm. Immunohistochemically, the tumour cells are positive for pan-cytokeratin, CK7, CK8, CK18, CK19, CEA, CA19-9, CA125, EMA, p53, surfactant apoprotein A and TTF-1. The tumour cells are negative for AFP, CD10, CK5/6, CK14, CK20, CK34ßE12, HMB45, p63, S100, SMA, synaptophysin and vimentin(27).
4. Clear cell variant of squamous cell carcinoma
Squamous cell carcinomas are tumours characterised by keratinisation and/or intercellular bridges. They arise from bronchial epithelial cells through squamous metaplasia/dysplasia. The 2004 WHO classification had 4 variants of squamous cell carcinoma, i.e. clear cell, small cell, papillary and basaloid(28). The 2015 WHO classification had no longer recommendation on this classification(10). Clear cell is considered to be more of a cellular change than a pattern and can occur in all histological categories of squamous cells. Immunohistochemistry shows positivity for CK5/6, p40 and p63, and negative for calponin, CK7, CK20, other myoepithelial markers like S100, SMA and TTF1(28).
5. Epithelial myoepithelial carcinoma
The tumour shows dual cell types, i.e. the duct/gland forming epithelial cells and the outer layer of myoepithelial cells. The epithelial cells are positive for CK7, while the myoepithelial cells are positive for CK5/6, p63, S100 and SMA(29).
6. Metastatic clear cell carcinoma
This tumour reveals specific histomorphology and immunohistochemical markers for their cells of origin(12).
Conclusion
In conclusion, clear cells and matrix-producing lung tumours should raise the suspicion of myoepithelial carcinoma. Since the available data regarding this rare entity is very minimum, big data case studies have to be undertaken for better treatment modalities and outcome of the disease.
Acknowledgement
This case report would not have been possible unless we were supported by the Departments of Nuclear Medicine, of Radiology and of Pathology at Aster MIMS, Kozhikode, Kerala, India and Dr Ajith Bhaskar, a pulmonologist in Kozhikode, Kerala, India.
References
- Sheldon WH. So-called mixed tumors of the salivary glands. Arch Pathol. 1943;35:1–20.
- Stromeyer FW, Haggitt RC, Nelson JF, Hardman JM. Myoepithelioma of minor salivary gland origin. Light and electron microscopical study. Arch Pathol. 1975;99:242–5.
- Strickler JG, Hegstrom J, Thomas MJ, Yousem SA. Myoepithelioma of the lung. Arch Pathol Lab Med. 1987;111:1082–5.
- Higashiyama M, Kodama K, Yokouchi H, et al., Myoepithelioma of the lung: report of two cases and review of the literature. Lung Cancer. 1998;20:47–56.
- Seifert G, Sobin LH. Myoepithelioma. World Health Organization International Histological Classification of Tumours: Histological Typing of Salivary Gland Tumours. 2nd ed. Berlin, Germany: Springer-Verlag; 1991. pp. 20-1.
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer, Lyon: 2015.
- Zhu F, Liu Z, Hou Y, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol 2013;8:1578–84.
- Shen C, Wang X, Che G. A rare case of primary peripheral epithelial myoepithelial carcinoma of the lung: case report and literature review. Medicine (Baltimore) 2016;95:e4371.
- Filisetti C, Russo T, Pansini A, Vella C, Viglio C, Riccipetitoni G. The First Reported Pediatric Case of Primary Myoepithelial Carcinoma Involving the Whole Lung: Surgical Radical Treatment and Prosthesis Implant. European J Pediatr Surg Rep. 2020;8(1):e52-e55.
- Mengoli MC, Longo FR, Fraggetta F, Cavazza A, Dubini A, Alì G, Guddo F, Gilioli E, Bogina G, Nannini N, Barbisan F, De Rosa N, Falconieri G, Rossi G, Graziano P. The 2015 World Health Organization Classification of lung tumors: new entities since the 2004 Classification. Pathologica. 2018;110(1):39-67.
- Khazeni K, LaBove H, Wilky B, et al., Myoepithelial carcinoma or epithelioid sarcoma – A rare diagnosis with poor prognosis. A case report and review of literature, Int J Surg Case Rep. 2018;49:239–243.
- Yoshida M, Yamashita D, Hamakawa H, Takahashi Y, Yasui H, Komatsu M, Ohbayashi C, Hara S. SMARCB1-deficient myoepithelial carcinoma of the lung: A case report. Human Pathology: Case Reports, Vol 21,2020:200414.
- Sekine I, Kodama T, Yokose T, et al. Rare pulmonary tumors-a review of 32 cases. Oncology 1998;55:431–4.
- Miura K, Harada H, Aiba S, et al. Myoepithelial carcinoma of the lung arising from bronchial submucosa. Am J Surg Pathol. 2000;24:1300–1304.
- Masuya D, Haba R, Huang C, Yokomise H. Myoepithelial carcinoma of the lung. Eur J Cardiothorac Surg. 2005;28:775–777.
- Tanahashi J, Kashima K, Daa T, et al. Pulmonary myoepithelial carcinoma resembling matrix-producing carcinoma of the breast: case report and review of the literature. APMIS. 2010;118:401–406.
- Sarkaria IS, DeLair D, Travis WD, et al. Primary myoepithelial carcinoma of the lung: a rare entity treated with parenchymal sparing resection. J Cardiothorac Surg. 2011;6:27.
- Hysi I, Wattez H, Benhamed L, et al. Primary pulmonary myoepithelial carcinoma. Interact Cardiovasc. Thorac Surg. 2011;13:226–228.
- Rosen LE, Singh RI, Vercillo M, et al. Myoepithelial carcinoma of the lung: a review. Appl Immunohistochem Mol Morphol. 2015;23:397–401.
- Wei J, Yuan X, Yao Y, et al. Primary myoepithelial carcinoma of the lung: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:2111–2116.
- Zhang Y, Li B, Hou J, et al. Primary myoepithelial carcinoma of the lung and 18F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2018;37:175–177.
- Zhou X, Yu M, Zhuo H, Zhang S. Primary pulmonary myoepithelial carcinoma in a young woman. Medicine (Baltimore). 2018;97(9):e0049.
- Kridis WB, Toumi N, Khanfir A, Hachicha M, Boudawara T, Frikha M. Primary pulmonary myoepithelial carcinoma in a child: An ambiguous entity. Lung India. 2015;32:497-9.
- Tsilimigras DI, Bakopoulos A, Ntanasis-Stathopoulos I, et al. Clear cell "sugar tumor" of the lung: Diagnostic features of a rare pulmonary tumor. Respir Med Case Rep. 2017;23:52-54.
- Weinreb I. Hyalinizing clear cell carcinoma of the salivary gland: a review and update. Head Neck Pathol. 2013;7:S20-S29.
- Jeffus SK, Gardner JM, Steliga MA, Shah AA, Stelow EB, Arnaoutakis K. Hyalinizing clear cell carcinoma of the lung: case report and review of the literature. Am J Clin Pathol. 2017;148(1):73-80.
- Goto T, Hada M, Oyama T. Lung adenocarcinoma with clear cell features producing carbohydrate antigen 19-9. Asian Cardiovascular and Thoracic Annals. 2015;23(8):985-987.
- Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18(9):2443-51.
- Arif F, Wu S, Andaz S, Fox S. Primary epithelial myoepithelial carcinoma of the lung, reporting of a rare entity, its molecular histogenesis, and review of the literature. Case Rep Pathol. 2012;2012:319434.