Thrombotic microangiopathy with Atypical Posterior Reversible Encephalopathy Syndrome (PRES) after kidney transplantation. A case report.
Wisit Liwlompaisan MD1, Adisorn Pathumarak MD1, Suchin Worawichawong MD2, Piyanuch Pootracool MD3, Vasant Sumethkul MD1.
1 Division of Nephrology, Department of Medicine, Ramathibodi Hospital,
Mahidol University, Bangkok, Thailand
2 Department of Pathology, Ramathibodi Hospital, Mahidol University, Bangkok,
Thailand 3Division of Vascular Surgery, Department of Surgery, Ramathibodi Hospital,
Mahidol University, Bangkok, Thailand
Corresponding author: Vasant Sumethkul MD,
Division of Nephrologgy, Department of medicine, Ramathibodi hospital,
270 Rama 6 Road, Bangkok 10400, Thailand,
E-mail. Vasant.sum@mahidol.ac.th.
Received 21 September 2015; Accepted 29 March 2016
ABSTRACT
Neurological manifestation of thrombotic microangiopathy (TMA) after renal transplantation is rare and difficult to be distinguished from other conditions. A 51-year-old female with ESRD of unknown etiology received deceased donor kidney transplantation from a 57-year-old donor. The immunosuppression included basiliximab induction, tacrolimus, mycophenolate mofetil (MMF) and prednisolone. She had delayed graft function requiring hemodialysis for 5 weeks. Kidney biopsy at 6th week showed acute tubular necrosis without evidence of rejection. Two months later she presented with alteration of mental status, psychomotor retardation, slow speech followed by generalized tonic-clonic seizure. Serum electrolyte was normal. The platelet count was 48,000 /ml and peripheral blood smear shows microangiopathic hemolytic anemia (MAHA) pattern. Tacrolimus level was 6.0 ng/ml. MRI brain showed multifocal symmetric hypersignal Fluid-Attenuated Inversion Recovery (FLAIR) lesion at bilateral medial thalami and subcortical area compatible with atypical posterior reversible encephalopathy syndrome (PRES). Serum creatinine rose to 6.49 mg/ dL and blood urea nitrogen was 65 mg/dL. She was diagnosed as TTP/HUS and treated with plasmapheresis. Repeated kidney biopsy showed TMA. Several weeks after treatments, the neurological symptoms was improved but requiring regular hemodialysis. We conclude that PRES can be a manifestation of TMA in kidney transplant recipient.
INTRODUCTION
Posterior Reversible Encephalopathy Syndrome (PRES) is a rare condition characterized by headache, confusion, visual disturbance, seizures altered mental status. Radiological findings on magnetic resonance imaging (MRI) are signs of edema of posterior portion particularly occipital and parietal areas, being commonly bilateral.(1) Thrombotic microangiopathy (TMA) can induce PRES by endothelial damage leading to coagulation cascade activation with increased vascular permeability and lead to perivascular edema. We report a case of a kidney transplant recipient who developed TMA after kidney transplantation (KT) and have predominated neurological manifestations compatible with PRES.
CASE REPORT
A 51-year-old female with ESRD of unknown etiology received deceased donor KT from a 57-year-old donor. The cold ischemic time was 22 hours. The degree of HLA mismatch was 2 of 6 and the recipient PRA was 3%. The dialysis vintage was 8 years. The immunosuppression included basiliximab induction, tacrolimus, mycophenolate mofetil (MMF) and prednisolone. She had delayed graft function (DGF) requiring hemodialysis for 3 weeks. On 4th week, the kidney function was improved. Urine output increased to about 1000-3000 ml per day and serum creatinine was 2.17 mg/dl. Transplant kidney biopsy at 6th week showed acute tubular necrosis without evidence of rejection. Two months after KT she presented with the change of mental status for a few days. She subsequently had psychomotor retardation, slow speech, myoclonus on both hands followed by generalized tonic-clonic seizure. Antiepileptic drugs were administrated by phenytoin and diazepam intravenously. Serum electrolyte was normal, the platelet count was 48,000 /ml, hemoglobin 10.9 g/dl and peripheral blood smear show schistocyte, polychromasia, and decrease platelet compatible with microangiopathic hemolytic anemia (MAHA) pattern. Tacrolimus level was 6.0 ng/ml. MRI brain showed multifocal symmetric hypersignal FLAIR lesion at bilateral medial thalami, bilateral hypothalami, bilateral optic tract, bilateral periventricular frontal horns and subcortical area compatible with posterior reversible encephalopathy syndrome (PRES) [figure 1]. Serum creatinine rose to 6.49 mg/dL, blood urea nitrogen (BUN) was 65 mg/dL and donor-specific antibody is negative. The diagnosis of TTP/HUS was given. Plasmapheresis was initiated accompany with hemodialysis. Tacrolimus was stopped. Repeated kidney biopsy showed fibrin thrombi, shrinkage of the glomerular tufts, without crescent. The arteries show endothelial swelling with foamy cells and focal intimal arteritis, arterioles show moderate hyaline and fibrin thrombi. The findings are compatible with thrombotic microangiopathy [figure 2]. The peritubular capillaries (PTC) show focal luminal leukocytes (less than 10% of cortical PTC) and the immunop-eroxidase staining for C4d is focally positive (10%). Several weeks after treatments, the neurological symptoms improved, TMA was in remission but required regular hemodialysis.
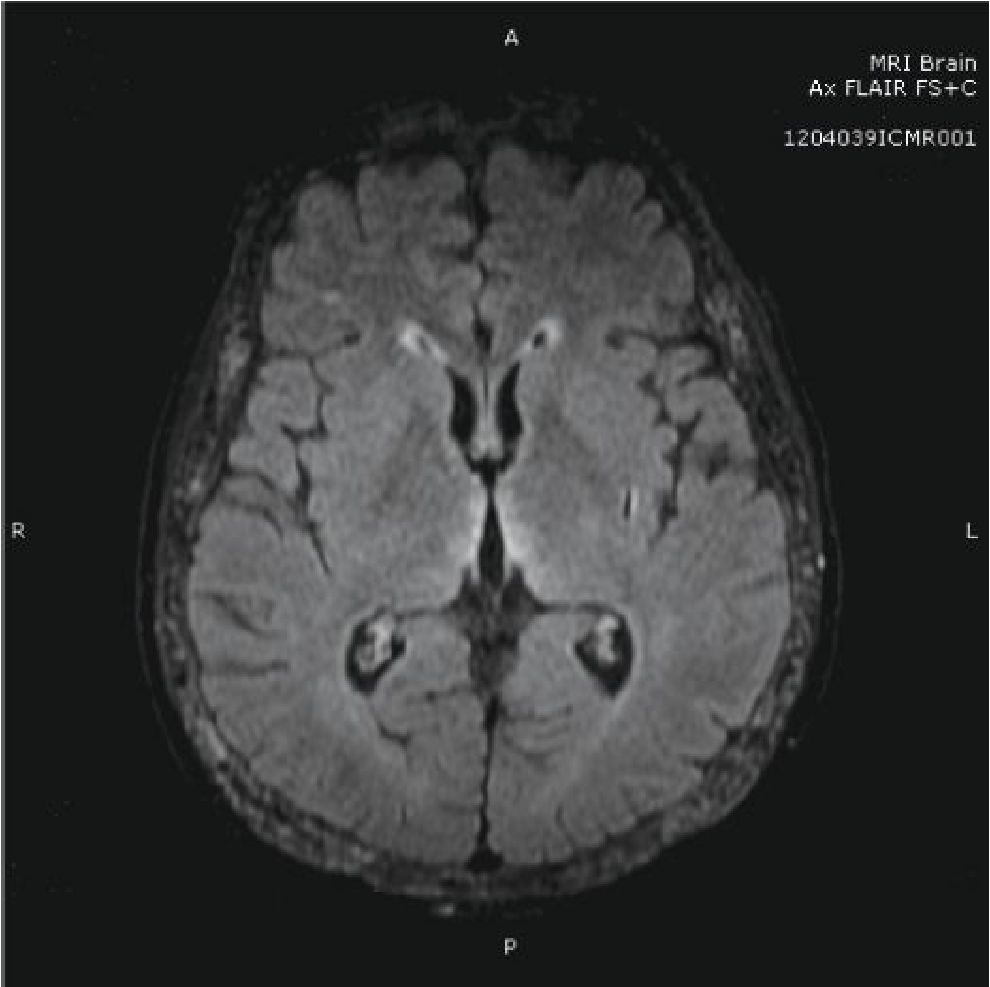
Figure 1 MRI brain showed multifocal symmetric hypersignal FLAIR lesion at bilateral medial thalami, bilateral hypothalami, bilateral optic tract, bilateral periventricular frontal horns and subcortical area
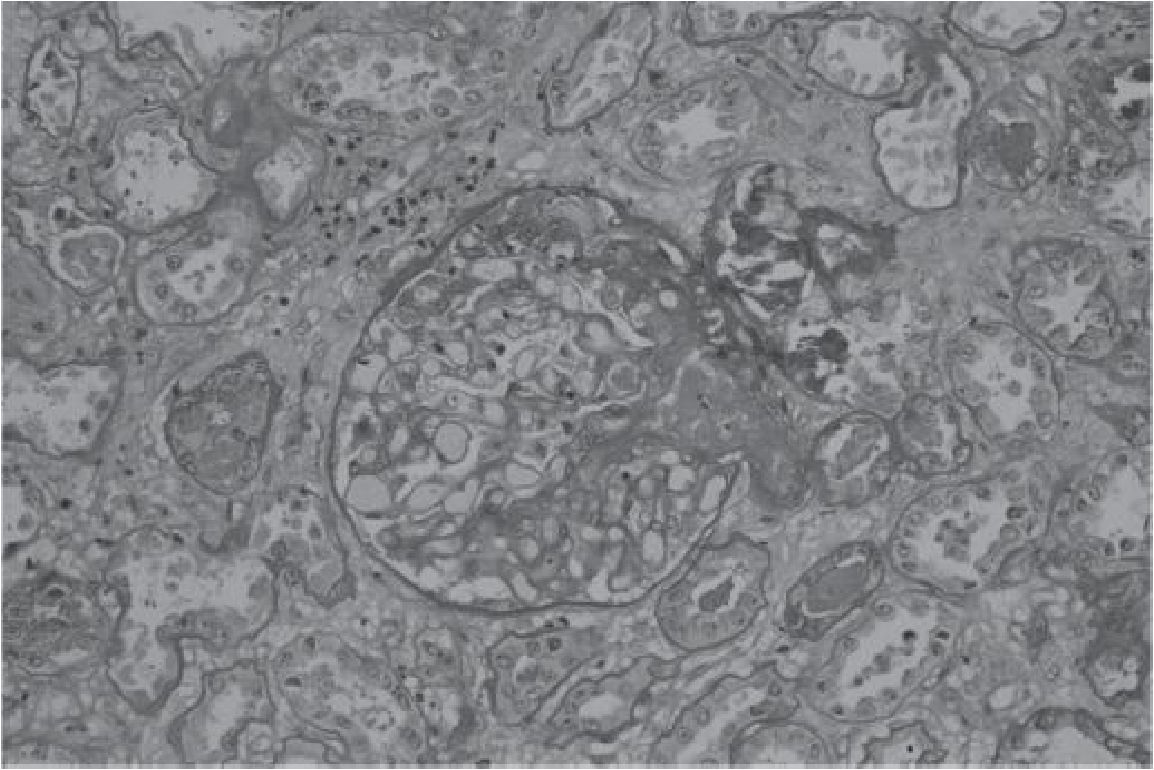
Figure 2 Renal pathology showed fibrin thombus in the afferent arteriole. The tubules show diffuse injury and necrosis.
DISCUSSION
Thrombotic microangiopathy (TMA) could be seen in liver or hematopoietic transplantation or calcineurin inhibitor used but unusual in KT. (7) In the native kidney, the incidence of TMA is around 2 cases/ 100,000 persons/year. Most cases presented in childhood and associated with Shiga-like toxin Escherichia coli. The remaining cases are atypical or non-diarrheal hemolytic uremic syndrome (aHUS). Progression to ESRD is as high as 50%. (2) Renal involvement of TMA is characterized by glomerular and arteriolar thrombosis, intracapillary stagnation of erythrocytes and red blood cell fragments, endothelial cell swelling and detachment from basement membrane, glomerular ischemia and onion skin hypertrophy of the arteriolar wall.(2) Posttransplant TMA may be de novo or recurrent in allograft TMA.(3) De novo TMA after KT may be triggered by acute antibody mediated rejection, viral infection or immunosuppressive drugs such as calcineurin inhibitors, mTOR inhibitors or antithymocyte globulin.(2,7) De novo TMA occurs in 4- 15 percent after KT, the graft survival was around 40 percent. Only 30 percent of the latter was renal limited TMA and the remaining had systemic symptoms. (2) Posttransplant PRES has been increasingly reported associated with immunosuppressive agents. (7,8,9) TMA and PRES shared the pathogenesis of endothelial injury that can be triggered by several factors such as hypertension, immunosuppressive drugs (cyclosporine or tacrolimus, or mTOR inhibitor) or acute antibody-mediated rejection. In our case, posttransplant TMA may be related with several factors mentioned above. The treatment of PRES is to stop the precipitating factors, airway support, and anti-convulsant agents when indicated. (1) The treatment of posttransplant TMA included reduction or withdrawal of CNI combine with plasma exchange. It is uncertain whether plasma exchange is imperative in the treatment of posttransplant TMA,(4) However, it is generally accepted that severe systemic TMA should be treated with plasma exchange. Post-transplant TMA may be associated with high mortality rate. The rate of graft loss ranged from 60 -100 %.(5, 6) We thus have shown in our case report that Posterior Reversible Encephalopathy Syndrome can be a manifestation of TMA in kidney transplant recipient.
REFERENCES
1. Aridon P RP, Mazzola MA. Reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura. Neurol Sci. 2011;32(3):469-72.
2. Noris M. RG. Thrombotic microangiopathy after kidney transplantation. American Journal of Transplantation. 2010;10:1517-23
3. Reynolds JC AL, Yuan CM, Abbott KC. Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 2003;42:1058-68.
4. Karthikeyan V PR, Shah V, Vera E, Venkat KK. . Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. . Am J Transplant 2003;3:1289-94.
5. Caires RA MI, Repizo LP. De novo thrombotic microangiopathy after kidney transplantation: clinical features, treatment, and long-term patient and graft survival. Transplant Proc. 2012;44(8):2388-90.
6. Ashman N CA, Dobbie H. Belatacept as maintenance immunosuppression for postrenal transplant de novo drug-induced thrombotic microangiopathy. Am J Transplant 2009;9:424-7.
7. Signh N, Bonham A, Fukui M. Immunosuppressive-associated leukoencephalopathy in organ transplant recipients. Transplantation 2000;69:467-72.
8. Besenski N, Rumboldt Z, Emovon O, Nicholas J, Kini S, Milutinovie J, et al. Brain MR imaging abnormalities in kidney transplantation. Am J Neuroradiol 2005; 26:2282-9.
9. Alxander S, David VG, Varughese S, Tamila-rasi, Jacob CK. Posteria reversible encephalopathy syndrome in a renal allograft recipient: A complication of immunosuppression?. Indian J Nephrol 2013;23:137-9


