A practical approach to undifferentiated tumors: A review on helpful markers and common pitfalls
Kanapon Pradniwat, MD, Jitsupa Treetipsatit, MD
Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University
Corresponding author: Jitsupa Treetipsatit, MD
Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University,
2 Prannok Road, Bangkok Noi, Bangkok 10700, Thailand Phone: 662-419-6520 Fax: 662-411-4260
E-mail address: jtreetipsatit@gmail.com
Received: 12 August 2013; Accepted 28 September 2013
Abstract
The term "undifferentiated" tumor is commonly used by pathologists when a tumor lacking evidence of lineage differentiation on the basis of routine light microscopic morphology is encountered. Immunohisto-chemistry plays an important role in elucidating the true nature of the undifferentiated tumor that is crucial for clinical management. Since undifferentiated round cell tumors can be mimicked by different types of tumors and often cause diagnostic problems especially in small biopsy specimen, the aim of this review is to provide a practical approach to select helpful immunohistochemical markers, a proposed algorithmic approach and pitfalls that may encountered.
Keywords: Undifferentiated tumors, Round cell tumors
Introduction
Pathologists usually use the term “undifferentiated” tumor when a tumor lacks histologic features which specify its lineage of differentiation. However, among these undifferentiated tumors, a large proportion of cases can be delineated by ancillary techniques namely immunohistochemistry. To cope with the expense, time-consumption and workloads, a systematic approach in using the appropriate immunohistochemical markers should be put in consideration. This article is aimed to provide: 1) a concise review on general approach for undifferentiated tumors as well as markers that are parts of the screening panels used in the recognition of the major lineages of the tumor; 2) a proposed algorithmic approach using immunohistochemistry; and 3) common pitfalls encountered when dealing with undifferentiated tumors with round cell morphology.
GENERAL APPROACH FOR UNDIFFERENTIATED TUMORS
The followings are practical steps of approach to the undifferentiated tumor.
Step 1: Categorize tumor according to morphologic appearances.
Undifferentiated tumors can be categorized according to morphologic appearances into 4 major groups:
1) Small round cell tumor - In this group, it comprises heterogeneous groups of tumors that are composed of relatively small, round to oval, closely packed undifferentiated cells with high nuclear- to-cytoplasmic ratio, scant cytoplasm, and round nuclei1,2
2) Large round cell/epithelioid cell tumor - The tumors belonged to this group are that of large round-oval to polygonal shape with a nesting or sheeting arrangement1.
3) Spindle cell tumor - The tumors that fall into this group are composed of spindle-shaped neoplastic cells with variable patterns of growth.
4) Pleomorphic tumor - A cellular component of these tumors shows high-grade sarcoma-like features with marked cellular pleomorphism and marked nuclear atypia.
Step 2: Determine a main lineage of differentiation.
Main lineages of tumor differentiation are listed as follow:
1) Epithelial tumors (carcinomas)
2) Germ cell tumors
3) Melanocytic tumors (malignant melanoma)
4) Hematopoietic and lymphoid tumors
5) Mesenchymal tumors (sarcomas).
Although morphologic clues such as a specific growth pattern and nuclear or cytoplasmic characteristics can guide to a diagnosis in some cases of undifferentiated tumors, ancillary studies especially immunohistochemistry are needed to determine a lineage of differentiation. Immunohis-tochemical studies are particularly helpful in small biopsy specimen by which morphologic assessment is limited. A screening panel of immunohisto-chemistry for major lineage differentiation often provides the first clue to the nature of an undifferentiated tumor.
Step 3: specify a diagnosis.
Once the main lineage of tumor differentiation is determined, one can proceed to make a much more specific diagnosis. In this step, clinical correlation as well as additional ancillary studies is needed.
Immunohistochemical markers for broad lineage DETERMINATION
Suggested screening markers for epithelial differentiation
• Cytokeratins (CKs)
Since the majority of carcinomas, either viable or extensively necrotic, will stain with CKs provided that an appropriate CK antibody is applied; this family of intermediate filaments is crucial in diagnostic immunohistochemistry for the identification of carcinomatous differentiation and specific carcinoma subtypes3-5. A large number of monoclonal antibodies that can be used on formalin-fixed paraffin-embedded tissues have been developed and are commercially available; those that play an important role in screening for epithelial differentiation are broad-spectrum CK antibodies6. Broad- spectrum CK antibodies that have been used more often in the published studies and might be helpful in diagnostic pathology are briefly discussed as follows:
Antibody cocktail AE1/AE3
This antibody comprises the mouse monoclonal antibody AE1 that recognizes the acidic (type I) CKs 10, 14, 15, 16, and 19, and AE3 that reacts with the basic (type II) CKs 1, 2, 3, 4, 5, 6, 7, and 87. Although the cocktail contains a wide variety of antibodies to important high-molecular weight (HMW) and low-molecular weight (LMW) CKs, it should be noted that this cocktail is not efficient in detecting CK8, which is the major CK in hepatocellular carcinoma and many other carcinomas5. Also it is inferior to a HMW antibody clone 34ßE12 (reacts with HMW CKs 1, 5, 10 and 14) in recognizing tumors with squamous differentiation5. Another drawback of this cocktail is that it does not react with CK18, which is expressed in many carcinomas5, 6. Thus, it is important to keep in mind that a negative staining for AE1/AE3 should not be regarded as sufficient evidence to rule out the possibility of carcinoma. In order to increase the sensitivity of the AE1/AE3 cocktail for detecting epithelial differentiation, addition of antibody clones that react with CK8 and 18 is suggested3, 6. Crossreaction with other intermediate filaments, especially glial fibrillary acidic protein (GFAP), can result in a false-positive staining result in glial tumors8.
Monoclonal antibody KL1
This broad-spectrum CK antibody is one of the most sensitive anti-keratin antibodies currently available for the detection of epithelial differentia- tion6. KL1 reacts with CKs 1, 2, 5, 6, 7, 8, 11, 14, 16, 17, and 18; therefore, it has an advantage over AE1/AE3 in detecting CK18. Similar to AE1/AE3, cross reaction with normal brain tissue and astrocytic tumors may be observed9.
Monoclonal antibody OSCAR
It is a newly developed broad-spectrum, anti-keratin mouse monoclonal antibody that reacts with CKs 7, 8, 18, and 19, as well as with other CKs not readily identified6. This antibody seems to have an advantage on the formerly mentioned broad- spectrum CK antibodies that it does not cross-react with normal brain tissue or gliomas6.
Antibody cocktail MAK-6
This cocktail antibody is composed of 2 mouse monoclonal antibodies: antibody KA4 (recognizes CKs 14, 15, 16 and 19) and antibody UCD/PR10.11 (reacts with CKs 8 and 18). According to Listrom et al10, MAK-6 reacts with all squamous cell carcinomas, and the majority of adenocarcinomas, transitional cell carcinomas, carcinoid tumors, and undifferentiated carcinomas. Similar to AE1/ AE3 and KL1, MAK-6 may show cross-reactivity with neural tissue11.
Monoclonal antibody CAM 5.2
CAM 5.2 is a mouse monoclonal antibody that primarily reacts with CK 8. Although it is technically not a pan-keratin antibody, it can be used as a screening marker for epithelial differentiation because CK 8 is widely expressed among epithelial tumors and it is highly sensitive for detecting CK 8 on formalin-fixed, paraffin-embedded tissues6.
Monoclonal antibody 34ßE12
It is sometimes referred to as keratin 903. This antibody reacts with HMW CKs 1, 5, 10 and 14. Strong and diffuse staining is observed in tumors with squamous epithelial differentiation3, 5, 6. Additionally, a wide variety of duct-derived carcinomas and mesotheliomas are noted to be positive for 34ßE123, 6. When combining with antibody to LMW CKs (e.g. CAM 5.2), 34ßE12 will increase sensitivity in detecting epithelial tumors5.
• Supplemental epithelial markers
These markers, though not specific to epithelial tumors, are useful in confirmation of epithelial differentiation when other lineages of differentiation are excluded and CKs are negative or focally positive. The supplement epithelial markers that might be useful in corroborating a diagnosis of epithelial tumor include carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), epithelial cell adhesion molecule (EpCAM) such as Ber-EP4 and MOC31, and claudin-4.
Suggested screening markers for malignant germ cell differentiation
• SALL4
SALL4 is a transcription factor required for the development and maintenance of embryonic stem cell pluripotency. This marker is relatively specific for either metastatic or primary germ cell tumors of different sites with up to 100% specificity and sensitivity in detecting seminoma, dysgerminoma, embryonal carcinoma and yolk sac tumor provided tumor cells demonstrate strong and diffuse (> 50%) nuclear positivity for the marker12-15. Comparing to placental alkaline phosphatase (PLAP), SALL4 provides superior sensitivity and specificity and is easier to interpret16. SALL4 is superior to OCT3/4 in detecting yolk sac tumor since OCT3/4 is usually negative in this tumor.
• Human chorionic gonadotropin (HCG)
In case that choriocarcinoma is in a list of differential diagnosis, addition of HCG to a screening panel is suggested16 since SALL4 is focally and weakly expressed or is not expressed at all in choriocarcinoma12-16.
Suggested screening markers for melanocytic differentiation
• S100 protein or SOX 10
S100 protein is regarded as a screening marker for melanoma due to its high (>95%) sensitivity for detecting melanoma in primary and metastatic sites1, 17. The characteristic staining pattern is strong and diffuse and occurs in the nucleus and the cell cytoplasm, but sometimes it is reported to be focal17. Despite its high sensitivity for melanoma detection, it should be noted that S100 protein is not specific because it may also be present in some carcinomas (especially those originating in the breast and salivary gland), some mesenchymal tumors (e.g. clear cell sarcomas, extraskeletal myxoid chondrosarcomas, rhabdomyosarcomas, leiomyosarcomas, synovial sarcomas, peripheral nerve sheath tumors, cartilaginous tumors, chordomas) and Langerhans cell histiocytosis.
SOX 10 is a transcription factor that participates in the late stage of neural crest cell formation, maintenance of multipotency of crest cells as a stem cell, and specification of derivative cell fates to schwannian and melanocytic destination18. The staining for SOX 10 is nucleus. It is one of the recent markers that has been recognized as being useful in the diagnosis of melanoma with the sensitivity comparable to S100 in detecting both primary and metastatic melanoma (>90%)17. It is believed that SOX 10 is superior to S100 protein as a screening marker for melanoma since it is negative in carcinomas and most soft tissue tumors apart from clear cell sarcomas, peripheral nerve sheath tumors (including neurofibromas, schwannomas, and malignant peripheral nerve sheath tumors), granular cell tumors, diffuse astrocytoma, rare cases of rhabdomyosarcomas, glomus tumors, and pleomorphic undifferentiated sarcomas18, 19. Also the positivity is usually observed in >50% of tumor cells in case of melanomas18.
• Specific markers for melanocytic differentiation: HMB-45, Melan A and Tyrosinase
These 3 markers are considered to be specific markers for melanocytic differentiation because they are monoclonal antibodies that specifically recognize either an important protein that plays an important role in melanosome formation (HMB-45 and Melan A) or principal enzyme required for the synthesis of melanin (tyrosinase). HMB-45, Melan A and tyrosinase have comparable sensitivity and specificity in detection of both primary cutaneous melanoma and metastatic melanoma with epithelioid morphology17. Despite their high specificity for melanoma detection, it should be kept in mind that these markers can be positive in clear cell sarcomas and various pigmented neuroectodermal tumors (e.g. melanotic schwannoma, melanotic neuroectodermal tumor of infancy)17. Perivascular epithelioid cell tumors (PEComa family), some ovarian steroid cell tumors, and renal cell carcinomas with the t(6;11)(p21;q12) translocation can also be positive for HMB-4517. Melan A clone A103 is observed to react with adrenal cortical tumors and sex-cord gonadal tumors whereas the M2-7C10 does not show reactivity with these tumors17.
It has been suggested that one or more melanocytic differentiation-specific markers should be additionally incorporated into the immunohisto- chemistry panel for undifferentiated tumor in order to confirm melanocytic lineage in case that the initial screening marker (either SOX 10 or S100 protein) is positive and markers for other lineages of differentiation are negative1,5.
Suggested screening markers for hematopoietic and lymphoid tumors
• Leukocyte common antigen (LCA) or CD45RB
This marker is considered to be a screening marker for hematopoietic and lymphoid neoplasms review on helpful markers and with high sensitivity (97%) and specificity (nearly 100%)20, 21. The characteristic pattern of staining is membranous. Despite its high sensitivity and specificity, lacking of LCA expression does not completely exclude hematopoietic and lymphoid tumors because there are some tumors that are negative for LCA (e.g. classical Hodgkin lymphomas, anaplastic large cell lymphomas, lymphoblastic lymphomas, plasma cell neoplasms) as well as there are variations in LCA expression in lymphoid and myeloid neoplasms.
• Additional markers for hematopoietic and lymphoid tumors that might be negative for LCA
• CD30 - CD30 should be included in the screening panel of undifferentiated tumors which large cell lymphomas including anaplastic large cell lymphoma and classical Hodgkin lymphoma are in a list of differential diagnosis5, 22 because these tumors are usually negative for LCA.
• CD43 - Since not all of myeloid neoplasms express LCA, addition of CD43 that is a more sensitive marker for myeloid neoplasm to the screening panel is suggested when a possibility of myeloid neoplasms cannot be excluded1, 22. In addition to detecting myeloid neoplasms, CD43 can serve as a pan T-cell marker and help detecting some T-cell lymphoid neoplasms that might be negative for LCA.
• CD138 - CD138 - In case that plasmablastic/ plasmacytic features are present, CD138 is needed for detection of tumors with plasmablastic/ plasma- cytic differentiation which are usually negative for LCA1, 23.
• TdT - TdT - Addition of TdT in the screening panel for small round cell tumors of a pediatric population is suggested for effective detection of lymphoblastic lymphoma/ leukemia1, 2.
• CD20, CD79a and/or Pax5 - Due to occasional loss of LCA expression in B-cell lymphomas, additional staining for one or more B-cell lineage specific markers (CD20, CD79a and/ or Pax5) is helpful in detecting B-cell lymphomas with loss of LCA expression when lymphoid neoplasm cannot be excluded22, 23.
Suggested screening markers for mesenchymal tumors
• Vimentin
Vimentin is the only intermediate filament characteristic of mesenchymal cells and present in virtually all sarcomas. However, it can be expressed in melanomas as well as in some lymphomas and carcinomas1.
• CD34
In the context of undifferentiated tumors with large round cell/ epithelioid cell features, CD34 is diagnostically useful in differentiating sarcomas with epithelioid features (e.g. epithelioid sarcoma, epithelioid angiosarcoma, epithelioid variant of gastrointestinal stromal tumor) from carcinomas24.
Suggested neural and neuroendocrine specific markers
• Synaptophysin and Chromogranin A
Synaptophysin and chromogranin A are helpful markers that should be included in an immuno- histochemistry panel for undifferentiated tumors with large round cell/ epithelioid cell morphology that a neuroendocrine tumor is in a list of differential diagnosis24. It is also important to keep in mind that clear cell sarcoma of the gastrointestinal tract is a distinct entity that is different from clear cell sarcoma of soft tissue and can be positive for these markers. In the context of small round cell tumors in a pediatric group, these markers are helpful in detecting tumors with neural differentiation such as Ewing’s sarcoma/ primitive neuroectodermal tumor, desmoplastic small round cell tumor and neuroblastoma1, 2, 24.
ALGORITHMIC APPROACH TO LINEAGE DIFFERENTIATION OF ROUND CELL UNDIFFERENTIATED TUMORS
A practical algorithmic approach to lineage determination of round cell undifferentiated tumors should be as follow:
• Large round cell/ epithelioid cell tumors
Since the differential diagnosis of the tumors in this group includes melanoma, hematopoietic and lymphoid malignancy, neuroendocrine tumors, poorly differentiated carcinomas and mesenchymal neoplasms with epithelioid feature. The initial battery of immunohistochemical markers should include a broad keratin (including both HMW and LMW CKs), S100 or SOX 10, LCA, SALL4 and vimentin. The algorithmic immunohistochemical approach is outlined in Figure 1.
When there is co-expression of vimentin with CK in the undifferentiated tumor with large round cell/ epithelioid cell features, the differential diagnosis is mainly carcinomas vs. sarcomas with vimentin/ CK co-expression or aberrant expression. Our initial approach is to consider whether CK expression is diffuse (> 50% of tumor cells) or focal (< 50% of tumor cells). If diffuse CK expression is noted and mesothelioma which has a distinct clinical manifestation is excluded, the differential diagnosis will include certain types of carcinomas that show vimentin co-expression (such as renal cell carcinoma, pulmonary adenocarcinoma, thyroid carcinoma, endometrial adenocarcinoma, and ovarian serous carcinoma) and some sarcomas including epitheloid sarcoma, malignant rhabdoid tumor, chordoma, and parachordoma/ mixed tumor of soft tissue. If carcinoma is initially suspected, additional stain-ings for tumor- or organ-specific markers (such as renal cell carcinoma (RCC) marker, thyroglobulin,

Figure 1 Algorithmic approach to undifferentiated tumors with large round cell/ epithelioid cell morphology. CK indicates cytokeratin; LCA, leukocyte common antigen; GCTs, germ cell tumors; and PEComas, perivascular epithelioid cell tumors.
* Neuroendocrine tumors include pheochromocytoma, paraganglioma and adrenal cortical tumor."
thyroid transcription factor 1 (TTF-1), WT1, etc.) may lead to the diagnosis. If the tumor is negative for tumor- or organ-specific markers, a possibility of sarcoma with vimentin/ CK co-expression should be raised and additional workups to specify the type of sarcoma should be performed. For an undifferentiated tumor with large round cell/ epithelioid features that focally expresses CK in addition to vimentin, there are two possibilities: a sarcomatoid carcinoma vs. a sarcoma with aberrant CK expression. Aberrancy of CK expression has been described in various sarcomas with epithelioid features, for example, epithelioid angiosarcoma (10%25), epithelioid malignant peripheral nerve
sheath tumor (<10%25), epithelioid leiomyosarcoma (<10%25), epithelioid rhabdomyosarcoma26, and epithelioid osteosarcoma27-30. In distinguishing between sarcomatoid sarcoma and sarcoma with aberrant CK expression, an additional panel of immunohistochemistry is required and should include markers that are usually positive in carcinomas but rarely positive in sarcomas and markers that are relatively specific to the aforementioned sarcomas that can show aberrant CK expression. Our markers of choice are p63 (mostly positive in carcinomas with rare positivity in some sarcomas31, 32), CD34, endothelial markers (e.g. CD31, factor VIII, D2-40, Friend leukemia integration 1 (FLI-1), ERG, etc.),
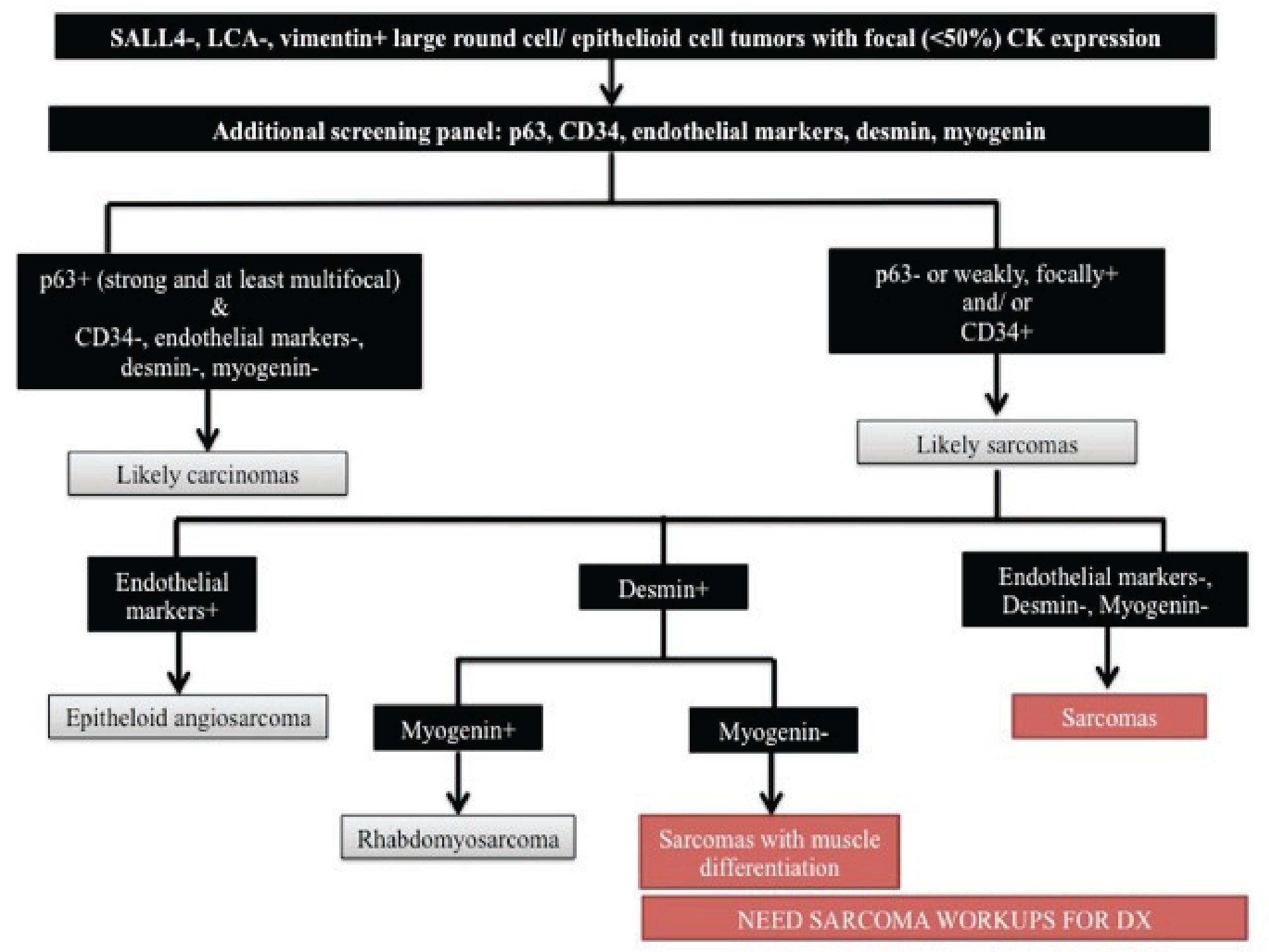
Figure 2 Algorithmic approach to SALL4-/ LCA-/ vimentin+ large round cell/ epithelioid cell tumors with focal CK expression. CK indicates cytokeratin and LCA, leukocyte common antigen
desmin, and myogenin. Figure 2 demonstrates an algorithmic approach to SALL4-/ LCA-/ vimentin+ large round cell/ epithelioid cell tumors with focal CK expression.
In case of an undifferentiated tumor with large round cell/ epithelioid cell features that lacks expression of SALL4, LCA and CK but shows positivity for vimentin and S100 protein or SOX10, it is important to include clear cell sarcoma of soft tissue and nerve sheath tumors with epithelioid features (such as epithelioid schwannoma, epithelioid peripheral nerve sheath tumor) in a list of differential diagnosis in addition to malignant melanoma. Additional stainings for markers that are specific for melanocytic differentiation (HMB- 45, Melan A and tyrosinase) help distinguish nerve sheath tumors from clear cell sarcoma. However,
these additional markers cannot distinguish clear cell sarcoma of soft tissue from melanoma. This distinction should be based on tumor morphology, location, lack of cutaneous involvement, and history of melanoma22, 33. Fluorescence in situ hybridization (FISH) analysis of the EWSR1 gene rearrangement is also useful for confirming the diagnosis of clear cell sarcoma22, 33.
For large round cell/ epithelioid cell undifferentiated tumors that are only positive for vimentin in the initial immunohistochemistry panel, differential diagnosis is quite broad and commonly includes LCA-negative hematopoietic and lymphoid tumors, neuroendocrine tumors (e.g. pheochromocytoma, paraganglioma, and adrenal cortical tumor), sarcomas and rare melanoma that is negative for S100 or SOX10. The first step of our approach to this group of tumors is to distinguish LCA-negative hematopoietic and lymphoid tumors from the others since these tumors need different clinical management. Immunohistochemistry markers of choice for detecting LCA-negative hematopoietic and lymphoid tumors with large cell morphology include CD30, CD43, CD138 and B-cell markers (CD20, CD79a and/or Pax5). Once a possibility of hematologic neoplasms is excluded, additional staining for neural and neuroendocrine specific markers (synap- tophysin and chromogranin A) should be performed in order to distinguish neuroendocrine tumors. If the tumor is negative for both synaptophysin and chro- mogranin A, the possibility of a S100-negative or SOX10-negative melanoma cannot be excluded and additional staining for melanocytic differentiation markers should be performed. When all of aforementioned tumors have been excluded, sarcoma is most likely and additional workups for subtyping sarcomas are needed. Figure 3 illustrates an algorithmic approach to large round cell/ epithelioid cell undifferentiated tumors that are only positive for vimentin in the initial immunohistochemistry panel.
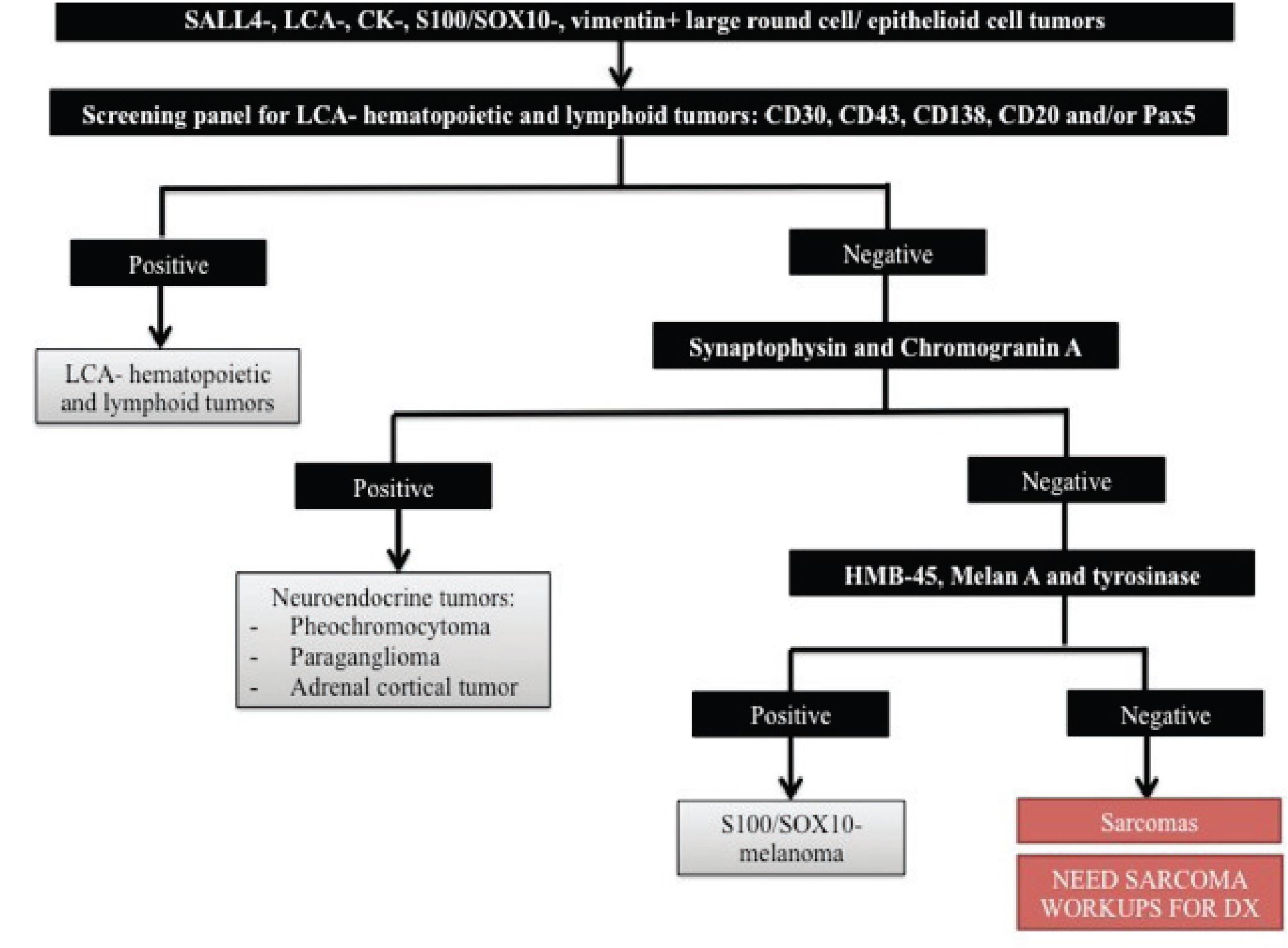
Figure 3 Algorithmic approach to large round cell/ epithelioid cell undifferentiated tumors that are only positive for vimentin in the initial immunohistochemistry panel. CK indicates cytokeratin and LCA, leukocyte common antigen
When all the markers in the initial screening panel including vimentin are negative, it is important to check for tumor cell viability. If tumor necrosis or poor tissue preservation is excluded, one should consider a possibility of LCA-/ vimentin- hematopoietic and lymphoid tumor, olfactory neuroblastoma and some types of sarcomas that can be vimentin- negative such as leiomyosarcoma, alveolar soft part sarcoma and rhabdomyosarcoma24, 25.
• Small round cell tumors
The name "small round cell tumor" is derived from the primitive, highly cellular nature of tumors in this group, which typically present a vast sea of dark-blue nuclei on hematoxylin-based stains2. Although this group of tumor is mainly composed of highly malignant neoplasms that occur in the pediatric age group; some adult tumors such as small cell carcinoma, variants of squamous cell carcinoma, poorly differentiated/ undifferentiated carcinomas, and small cell variant of malignant melanoma are also included into the differential diagnosis of small round cell tumor considering similarity in the morphologic findings.
Initial consideration of our approach to undifferentiated tumors with small round cell features is the age of patient. If the tumor is of an adult patient, it is essential to exclude adult tumors with small round cell features first before considering a workup for small round cell tumors of pediatric population. An initial immunohistochemistry panel for approach to pediatric small round cell tumors should include CK, vimentin, synaptophysin, chromogranin A, desmin, WT-1 (using antibodies that recognize C-terminal region of WT-1), LCA, CD43 and TdT. An outlined algorithmic approach is illustrated in Figure 4.
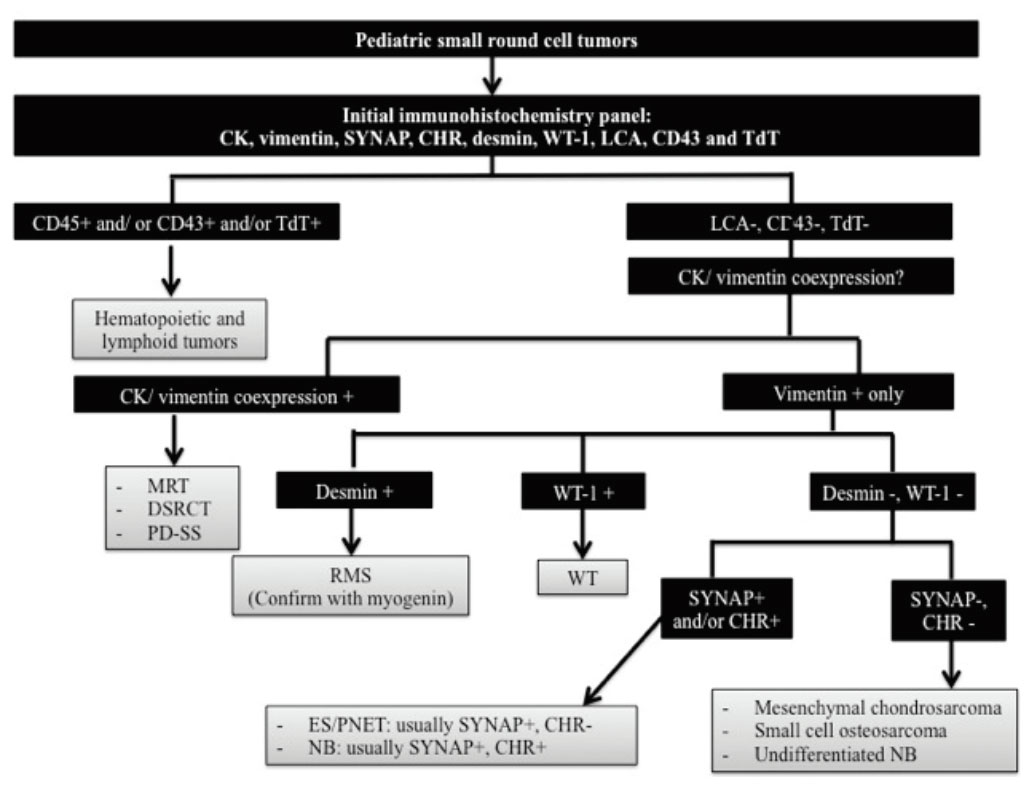
Figure 4 Algorithmic approach to pediatric small round cell tumors. CK indicates cytokeratin; SYNAP, synaptophysin; CHR, chromogranin A; LCA, leukocyte common antigen; MRT, malignant rhabdoid tumor; DSRCT, desmoplastic small round cell tumor; PD-SS, poorly differentiated synovial sarcoma; RMS, rhabdomyosarcoma; WT, Wilms tumor; ES/PNET, Ewing sarcoma/ primitive neuroectodermal tumor; and NB, neuroblastoma.
For a group of pediatric small round cell tumors that show co-expression of CK and vimentin, a next step of our approach is to evaluate reactivity of the tumor cells with desmin and WT-1 as well as performing additional staining for INI-1. If the tumor cells show reactivity with desmin and WT-1 in addition to CK and vimentin, desmoplastic small round cell tumor (DSRCT) is most likely. The diagnosis can be confirmed by additional FISH or molecular genetic analysis for EWS rearrangement. Loss of INI-1 expression in a tumor of an infant or a young child showing rhabdoid morphology, co-expression of CK and vimentin, and negativity for desmin and myogenin suggests the diagnosis of malignant rhabdoid tumor (MRT). Due to lacking specific diagnostic immunohistochemical marker, poorly differentiated synovial sarcoma (PD-SS) should be suspected after excluding other small round cell tumors in this group and establishment of the diagnosis need FISH or molecular genetic analysis for SYT-SSX fusion1, 22, 34.
Differential diagnosis of a small round cell tumor that expresses synaptophysin and/or chro-mogranin A in addition to vimentin in the initial screening panel includes Ewing sarcoma/ Primitive neuroectodermal tumor (ES/PNET) and neuroblastoma (NB). Besides characteristic clinical features (e.g. patient's age, detection of increased catecholamine metabolites in the serum and urine), additional stainings for CD99 and CD56 are helpful in distinguishing between these two diseases. In this context, a CD99+/ CD56- profile supports the diagnosis of ES/PNET while a CD99-/ CD56+ profile suggests the diagnosis of NB1, 2.
In the small round cell group, there are some tumors that are vimentin-positive only, for example, mesenchymal chondrosarcoma, small cell osteosarcoma, and undifferentiated NB. Identification of a well-formed hyaline cartilage component besides the undifferentiated small round cell component is diagnostic for mesenchymal chondrosarcoma. However, small biopsy specimens may lack of the well-formed cartilaginous component and distinction from other small round cell tumors may be difficult. Additional staining for immunohistochemical markers of chon-droid differentiation such as collagen type II and SOX-9 can be helpful in confirming the diagnosis under this situation35. For small cell osteosarcoma, distinction from other small round cell tumors essentially relies on the presence of osteoid production because of the lack of a specific immunoprofile for osteosarcoma1. In case that undifferentiated NB is suspected, correlation with clinical findings as well as additional staining for PGP9.5 (a neural marker that are usually positive in undifferentiated NB) might be needed for establishing the diagnosis36.
Although these algorithms are supposed to be a guiding map to the diagnosis of round cell undifferentiated tumors, it should be noted that each tumor should best be approached by an individually tailored selection of markers which appear most likely to the pathologist to yield information for the diagnosis.
COMMON PITFALLS THAT MIGHT BE ENCOUNTERED WHEN APPROACHING TO UNDIFFERENTIATED TUMORS WITH ROUND CELL MORPHOLOGY.
The following is a list of common pitfalls that we have seen in our daily practice:
• Inadequate screening markers for major lineage determination of the tumor
Since the differential diagnosis of undifferentiated tumors with round cell morphology is vast, a panel approach with adequate markers to appropriately address all the differential diagnostic possibilities is necessary. Many diagnostic errors happen because too few markers are performed.
• Lacking awareness of important exceptions for the so-called "specific" markers
It is important to always keep in mind that there are exceptions to everything. The diagnosis should not be based solely on reactivity of single marker. Overall assessment of the immunoprofile of a given tumor and correlation with morphological findings and relevant clinical information are keys to the correct diagnosis.
• "Paranormal" phenomena - an aberrant immu-nophenotype, a tumor with variation in antigen expression, and a tumor with heterologous differentiation
These "paranormal" phenomena occasionally occur and always lead to confusion for diagnostic pathologists. Awareness of this fact in the tumor of interest and thorough workups to exclude possibilities of other diagnoses are important to avoid the confusion and diagnostic errors.
• Technical factor that affects antigenicity of the tissue
Examples of such a situation include an increase in expression of CKs in a frozen-sectioned or alcohol/ acetone-fixed non-epithelial tumor comparing to a formalin-fixed tumor and a decrease in reactivity of nuclear-staining markers in a tissue that is decalcified in an acid solution.
REFERENCES
1. Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: true identity by immunohistochemistry. Archives of pathology & laboratory medicine 2008; 132(3):326-48.
2. Parham DM. Small Round Cell Tumors. In: Miettinen M (ed). Modern Soft Tissue Pathology: Tumors and Non-neoplastic conditions, Cambridge University Press: New York, 2010, pp 896-929.
3. Bhargava R, Dabbs DJ. Immunohistology of metastatic carcinomas of unknown primary. In: Dabbs DJ (ed). Diagnostic Immunohistochem-istry: Theranostic and Genomic Applications, 3rd edn, Saunders Elsevier: China, 2010, pp 206-55.
4. Judkins AR, Montone KT, LiVolsi VA, van de Rijn M. Sensitivity and specificity of antibodies on necrotic tumor tissue. American journal of clinical pathology 1998; 110(5):641-6.
5. Miller RT. Immunohistochemical Approach to "Undifferentiated" Tumors. American Academy of Oral and Maxillofacial Pathology Annual Meeting: San Juan, Puerto Rico, 2011.
6. Ordonez NG. Broad-spectrum immunohisto-chemical epithelial markers: a review. Human pathology 2013.
7. Cooper D, Schermer A, Sun TT Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Laboratory investigation; a journal of technical methods and pathology 1985; 52(3):243-56.
8. Kriho VK, Yang HY, Moskal JR, Skalli O. Keratin expression in astrocytomas: an immu-nofluorescent and biochemical reassessment. Virchows Archiv : an international journal of pathology 1997; 431(2):139-47.
9. Franke FE, Schachenmayr W, Osborn M, Altmannsberger M. Unexpected immunoreac-tivities of intermediate filament antibodies in human brain and brain tumors. The American journal of pathology 1991; 139(1):67-79.
10. Listrom MB, Dalton LW. Comparison of keratin monoclonal antibodies MAK-6, AE1:AE3, and CAM-5.2. American journal of clinical pathology 1987; 88(3):297-301.
11. Cosgrove MM, Rich KA, Kunin SA, Sherrod AE, Martin SE. Keratin intermediate filament expression in astrocytic neoplasms: analysis by immunocytochemistry, western blot, and northern hybridization. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 1993; 6(3):342-7.
12. Cao D, Humphrey PA, Allan RW. SALL4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009; 115(12):2640-51.
13. Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. The American journal of surgical pathology 2009; 33(7):1065-77.
14. Liu A, Cheng L, Du J, et al. Diagnostic utility of novel stem cell markers SALL4, OCT4, NANOG, SOX2, UTF1, and TCL1 in primary mediastinal germ cell tumors. The American journal of surgical pathology 2010; 34(5):697-706.
15. Mei K, Liu A, Allan RW, et al. Diagnostic utility of SALL4 in primary germ cell tumors of the central nervous system: a study of 77 cases. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2009; 22(12):1628-36.
16. Rabban JT, Zaloudek CJ. A practical approach to immunohistochemical diagnosis of ovarian germ cell tumours and sex cord-stromal tumours. Histopathology 2013; 62(1):71-88.
17. Ordonez NG. Value of melanocytic-associated immunohistochemical markers in the diagnosis review on helpful markers and of malignant melanoma: a review and update. Human pathology 2013.
18. Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. The American journal of surgical pathology 2008; 32(9):1291-8.
19. Karamchandani JR, Nielsen TO, van de Rijn M, West RB. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Applied immunohis-tochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry 2012; 20(5):445-50.
20. Kurtin PJ, Pinkus GS. Leukocyte common antigen--a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Human pathology 1985; 16(4):353-65.
21. Michels S, Swanson PE, Frizzera G, Wick MR. Immunostaining for leukocyte common antigen using an amplified avidin-biotin-peroxidase complex method and paraffin sections. A study of 735 hematopoietic and nonhematopoietic human neoplasms. Archives of pathology & laboratory medicine 1987; 111(11):1035-9.
22. Heim-Hall J, Yohe SL. Application of immunohistochemistry to soft tissue neoplasms. Archives of pathology & laboratory medicine 2008; 132(3):476-89.
23. Boyd SD, Natkunam Y, Allen JR, Warnke RA. Selective immunophenotyping for diagnosis of B-cell neoplasms: immunohistochemistry and flow cytometry strategies and results. Applied immunohistochemistry & molecular morphology : AIMM / official publication of the Society for Applied Immunohistochemistry 2013; 21(2):116-31.
24. Miettinen M. Immunohistochemistry of Soft Tissue Tumors. In: Miettinen M (ed). Modern Soft Tissue Pathology: Tumors and Non-neoplastic Conditions, Cambridge University Press: China, 2010, pp 44-104.
25. Wick MR, Hornick JL. Immunohistology of Soft Tissue and Osseous Neoplasms. In: Dabbs DJ (ed). Diagnostic Immunohistochemistry: Theranostic and Genomic Applications, 3rd edn, Saunders Elsevier: China, 2010, pp 83-136.
26. Jo VY, Marino-Enriquez A, Fletcher CD. Epithelioid rhabdomyosarcoma: clinicopatholog-ic analysis of 16 cases of a morphologically distinct variant of rhabdomyosarcoma. The American journal of surgical pathology 2011; 35(10):1523-30.
27. Hasegawa T, Hirose T, Seki K, et al. Histological and immunohistochemical diversities, and proliferative activity and grading in osteosarcomas. Cancer detection and prevention 1997; 21(3):280-7.
28. Hasegawa T, Shibata T, Hirose T, Seki K, Hizawa K. Osteosarcoma with epithelioid features. An immunohistochemical study. Archives of pathology & laboratory medicine 1993; 117(3):295-8.
29. Kramer K, Hicks DG, Palis J, et al. Epithelioid osteosarcoma of bone. Immunocytochemi-cal evidence suggesting divergent epithelial and mesenchymal differentiation in a primary osseous neoplasm. Cancer 1993; 71(10):2977-82.
30. Okada K, Hasegawa T, Yokoyama R, Beppu Y, Itoi E. Osteosarcoma with cytokeratin expression: a clinicopathological study of six cases with an emphasis on differential diagnosis from metastatic cancer. Journal of clinical pathology 2003; 56(10):742-6.
31. Jo VY, Fletcher CD. p63 immunohistochemical staining is limited in soft tissue tumors. American journal of clinical pathology 2011; 136(5):762-6.
32. Lewis JS, Ritter JH, El-Mofty S. Alternative epithelial markers in sarcomatoid carcinomas of the head and neck, lung, and bladder-p63, MOC-31, and TTF-1. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2005; 18(11):1471-81.
33. Miettinen M. Neuroectodermal Tumors: Melanocytic, Glial, and Meningeal neoplasms. In: Miettinen M (ed). Modern Soft Tissue Pathology: Tumors and Non-neoplastic conditions, Cambridge University Press: China, 2010, pp 724-54.
34. Tsokos M, Alaggio RD, Dehner LP, Dickman PS. Ewing sarcoma/peripheral primitive neuroectodermal tumor and related tumors. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 2012; 15(1 Suppl):108-26.
35. Cates JM, Coffin CM. Extraskeletal cartilaginous, osseous, and chordoid tumors in children and adolescents. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 2012; 15(1 Suppl):255-66.
36. Conran RM, Chung E, Dehner LP, Shimada H. The Pineal, Pituitary, Parathyroid, Thyroid, and Adrenal glands. In: Stocker JT, Dehner LP, Husain AN (eds). Stocker & Dehner's Pediatric Pathology, 3rd edn, Lippincott Williams & Wilkins: China, 2011.


