Pathological assessment of activity and chronicity indices in lupus nephritis patients
Thararat Prasanwong1, Nutthaporn Laoharojvongsa1, Kornwipa Pongpanich2, Bancha Satirapoj2 and Mongkon Charoenpitakchai3*
1 The Third-Year Anatomical Pathology Resident, Anatomical Pathology Division,
Army Institute of Pathology, Bangkok, Thailand
2 Nephrology Unit, Medicine Division, Phramongkutklao Hospital, Bangkok, Thailand
3 Department of Pathology, Phramongkutklao College of Medicine, Bangkok, Thailand
Correspondence to:
Lieutenant Colonel Associate Professor Dr Mongkon Charoenpitakchai,
Department of Pathology, Floor 6, Her Royal Highness Princess Bejaratana Building,
Phramongkutklao College of Medicine, 317 Rajavithi Road, Rajadevi, Bangkok 10400 Thailand.
Telephone: +66 (0) 86 562 2393 Fax: +66 (0) 2 354 7791
Email: mongkon.char@pcm.ac.th, mongkon@pcmpathology.org, dr.cmongkon@gmail.com
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Abstract
Lupus nephritis (LN) is one of a major complication of systemic lupus erythematosus (SLE). Ten percent of patients with LN will develop end-stage renal disease (ESRD). Renal biopsy is the gold standard for diagnosis of LN and evaluate activity and chronicity of disease to predict renal function outcome. Activity and chronicity indices by National Institutes of Health (NIH) provide prognostic value and treatment guidance, however these indices are poor intra and interobserver reproducibility to be used as therapeutic guides or as prognosticators. The aim of this retrospective study was using individual morphologic variables is easier to understand and apply in clinical practice for predicting renal outcome. This retrospective study was enrolled 38 patients with biopsy proven LN class III and IV seen over 3-year period. The demographic, clinical and laboratory data were obtained at the time of biopsy. Activity and chronicity indices were calculated and correlation between outcome parameters and the histological findings were investigated. Thirty-eight cases of LN were evaluated, of which 71% had LN class IV. The mean age was 29.63 ± 9.56 years, and 84% were females. The mean scores of activity index (AI) (NIH), chronicity index (CI) (NIH), modified NIH AI were 7.97, 2.79, and 6.47, respectively. Serum creatinine and eGFR correlated significantly with all indices as well as haematuria showed significant correlation with all indices except the chronicity index. Serum creatinine level was the strongest clinical parameter determining outcome. Urine protein to creatinine ratio (UPCR) showed limited correlation with leucocyte infiltration (r = 0.399, p = 0.013). In activity index, correlations with serum creatinine/estimated glomerular filtration rate (eGFR) were strongest with the interstitial infiltration (r = 0.557, p = 0.001) and fibrinoid necrosis/cellular crescent (r = 0.466, p = 0.003). In the chronicity index, correlations with serum creatinine/eGFR were strongest with glomerulosclerosis (r = 0.587, p = 0.001) and interstitial fibrosis (IF)/tubular atrophy (TA) (r = 0.448, p = 0.005). The eGFR was significantly decreased (less than 60 mL/min/1.73 m2), independently with these pathologic lesions, including presence of endocapillary hypercellularity ≥ 50% of total glomeruli, presence of subendothelial hyaline deposits ≥ 25% of total glomeruli, presence of fibrinoid necrosis/cellular crescent ≥ 25% of total glomeruli, presence of glomerulosclerosis ≥ 25% of total glomeruli, presence of fibrous crescent ≥ 5% of total glomeruli, IF/TA ≥ 10% of cortical area, and presence of adhesion to bowman's capsule ≥ 25% of total glomeruli, respectively. Base of these findings, we suggest the presence of any of the histological features of the AI (endocapillary hypercellularity, cellular/fibrocellular crescent and/or necrosis) reportedly defines patients at risk of developing renal failure. Similarly, the presence of any of the histological features of the CI (glomerulosclerosis, interstitial fibrosis and tubular atrophy) reportedly defines patients at risk of developing renal failure. In conclusion, modified NIH AI showed better correlation with clinical and outcome parameters as compared to the standard AI and CI scores, however these current scoring of AI and CI for LN exhibit poor interpathologist agreement. We suggest it could be improved by using individual morphologic variables that are easier to be performed in routine clinical practice for predicting renal outcome.
Keywords: activity index; chronicity index; lupus nephritis; renal biopsy; renal outcome
Introduction
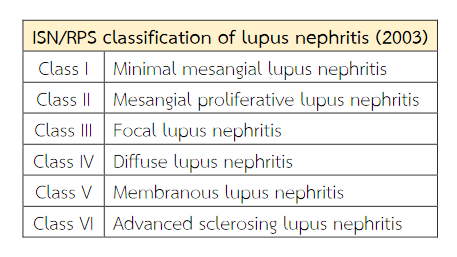
Lupus nephritis (LN) is one of a major complication of systemic lupus erythematosus (SLE). Ten percent of patients with LN will develop end-stage renal disease (ESRD)(1). The various clinical presentations are recognised in patients with lupus nephritis, ranging from mild asymptomatic to rapidly progressive glomerulonephritis(2,3) and usually correlating with the histologic type of lesion. The renal biopsies play an important role in the confirm diagnosis, evaluate disease activity, determine prognosis and management of patients with lupus nephritis (LN)(4,5). Classification of the renal pathology of lupus patients is based on light microscopic changes combined with immunofluorescence microscopy (IF) and electron microscopy(6). The diagnosis needs criteria in accordance with the 2003 International Society of Nephrology and Renal Pathology Society (ISN/RPS) classification into six different classes based on quantitative assessment of histological lesions(7) (Table 1). Parameters of activity and chronicity should be described in accordance with activity and chronicity index by National Institute of Health modified by Austin et al.(8) (Table 2).
Table 1 Abbreviated International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of lupus nephritis (2003).
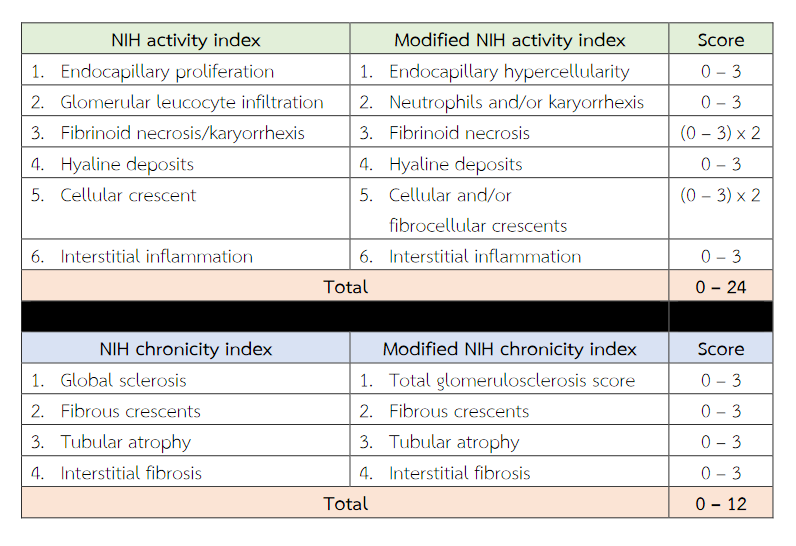
Activity Index (Al) is useful in assessing activity of LN. It consisted of the following items considered to represent measures of active lupus nephritis (endocapillary hypercellularity, glomerular leucocyte infiltration, subendothelial hyaline deposits, fibrinoid necrosis/karyorrhexis, cellular crescents and interstitial inflammation). These are scored from 0 to 3 depending on severity except fibrinoid necrosis/karyorrhexis, cellular crescents which assigned score was weighted by a factor of two(2) because such lesions were considered to be disproportionately severe(9). The maximum score was 24 points for the activity Index. Chronicity Index (Cl) is useful in assessing chronicity of LN. It consisted of the following items considered to represent measures of chronic irreversible lupus nephritis (glomerular sclerosis, fibrous crescents, tubular atrophy and interstitial fibrosis). These were semiquantitatively graded on a scale of 0, 1, 2 or 3. The maximum score was 12 points for the chronicity Index(8,9). The activity index (AI) represents the degree of inflammatory injury to renal parenchyma and generally comprises lesions that may be response to immunosuppressive therapy. The chronicity index (CI) represents the degree of chronic damage the kidney, and generally comprises lesions that are associated with refractoriness to aggressive therapy(10). Studies at the NIH correlated both a high activity index (score > 12) and high chronicity index (score > 4) with a poor 10-year renal survival rate. These provides useful information about the efficacy of therapy and the relative degree of reversible versus irreversible lesions(11). Wernick et al. compared the reproducibility in a setting of four community hospitals and one university medical centre(12). They found that the activity and chronicity indices were only moderately reproducible in a non-referral setting. Cecile Grootscholten et al. revealed that five specialised nephropathologists scored 126 biopsies from 87 patients with biopsy-proven proliferative LN. They found that there was a wide range of the agreement(13). The activity index for LN showed good [Intraclass correlation coefficient (ICC) = 0.716] and the chronicity index showed moderate (ICC = 0.494) interobserver agreement. Schwartz et al. studied the comparison of the activity (AI) and chronicity indices (CI) in the renal biopsies calculated by different pathologists and concluded that these indices are too subjective to be used as therapeutic guides or as prognosticators(14).
Gary S Hill et al. developed a new morphologic index for the evaluation of renal biopsies in lupus nephritis, comprised four elements: Glomerular Activity Index (GAI), Tubulointerstitial Activity Index (TIAI), Chronic Lesions Index, interstitial fibrosis index (IFI)(15). The Biopsy Index showed better correlations with clinical and outcome parameters than the standard AI and CI and other similar indices but this schema is very complex and its reproducibility has not been demonstrated. The international nephropathology working group in Leiden, Netherlands, in 2016 re-evaluation of activity and chronicity. In the modified NIH activity index, they link the presence of karyorrhexis to neutrophil infiltration and modified fibrinoid necrosis into a stand-alone. These were semiquantitatively graded on a scale of 0, 1, 2 or 3 (< 25%, 25 – 50% or > 50% of glomeruli, respectively)(16). However, the semiquantitative system for grading and scoring for each morphologic various lesions for assessing activity and chronicity index of both NIH and modified NIH scoring system exhibits poor interpathologist agreement(18) and it is subject to interobserver variability. The aim of this retrospective study was using individual morphologic variables is easier to understand and apply in clinical practice for predicting renal outcome.
Table 2 National Institutes of Health (NIH) and modified NIH lupus nephritis activity and chronicity scoring system.
Materials and Methods
Selection of patients:
We searched the pathology database to identify native renal biopsies of 38 patients from the archives of Army Institute of Pathology, Bangkok, Thailand from the period of 2016 to 2018 were evaluated. The patients fulfilled the revised American College of Rheumatology (ACR) criteria for SLE(17) as determined by their physicians. Renal biopsy confirmed lupus nephritis cases were classified as class III and IV according to the 2003 ISN/RPS LN classification(7).
Clinical and laboratory data:
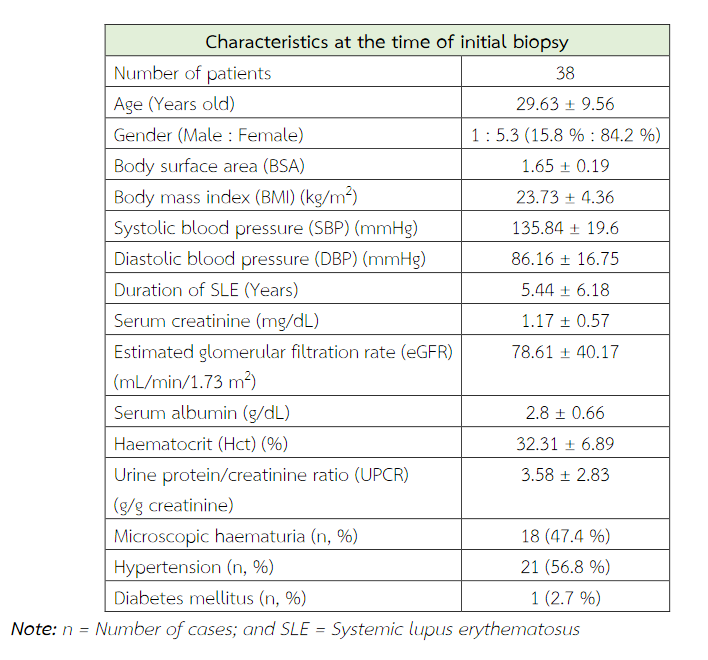
The following clinical parameters were evaluated at the time of each biopsy, i.e. age, sex, body weight, height, body surface area (BSA), body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), underlying diseases, ISN/RPS classification of lupus nephritis, duration of SLE, serum creatinine, estimated glomerular filtration rate (eGFR), haematocrit (Hct), serum albumin, urine protein/creatinine ratio (UPCR), haematuria and previous immunosuppressive treatments at the time of kidney biopsy were obtained from the patient records.
Pathological data:
Standard light microscopy (LM), sectioned with 2 micrometres thickness and the staining included haematoxylin and eosin stain, periodic acid-Schiff stain, and Jones methenamine silver stain was reviewed; the information collects include ISN/RPS classification of lupus nephritis and presence or absence of these features (endocapillary hypercellularity, subendothelial hyaline deposits, neutrophils infiltration, karyorrhexis, fibrinoid necrosis, cellular/fibrocellular crescents, interstitial infiltration, glomerular sclerosis, fibrous crescent, tubular atrophy, interstitial fibrosis and adhesion to Bowman's capsule). Adequate renal biopsy samples for histological diagnosis, including at least 5 glomeruli. The immunofluorescence (IF) images in computer files were reviewed in all cases; the information collects location of immunofluorescence staining, intensity of each staining (IgG, IgA, IgM, C3 and C1q) with the degree of intensity of 0 (negative), trace, 1, 2 and 3. All renal biopsies were reviewed by a Thai board-certified pathologist blinded to the clinical data. Six outcome parameters were measured, i.e. serum creatinine, estimated glomerular filtration rate (eGFR), Hct, serum albumin, urine protein/creatinine ratio (UPCR) and microscopic haematuria.
Statistical analysis:
All continuous values were expressed as mean ± standard deviation (SD) and categorical variables were presented as percentage. Pearson's correlation and Chi square tests were used to compare frequency variables and correlation among different variables. Receiver Operating Characteristic (ROC curve) to determine a cutoff value. Data were analyzed by Stata software (Stata Corp. 2011. Stata Statistical Software: Release 12. College Station, TX: Stata Corp LP). The p-value of less than 0.05 was assumed to be significant.
Ethical Statement:
This study was reviewed by the Institutional Review Board of Royal Thai Army Medical Department (S003b/62_Exp).
Results
Baseline data of patients with lupus nephritis:
A total of 38 patients were included in this study with age ranged from 16 to 56 years. Thirty-two patients (84.2%) were female. Clinical data at biopsy time is shown in Table 3. According to International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification, 11 patients are classified as class III (28.9 %) and 27 (71 %) cases are class IV (Table 4).
Comparison with NIH activity and chronicity indices with other indices:
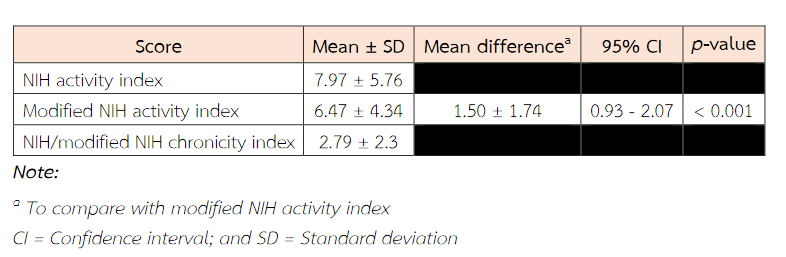
The mean score (± SD) for the activity index was 7.97 ± 5.76, and the mean score for the chronicity index was 2.79 ± 2.3 (Table 5). Ranges for the activity and chronicity indices were 0 to 19 and 0 to 7, respectively. For the activity index, 28.9 % were scored in the high-risk range of 12 or greater(9). For the chronicity index, 36.9% of scores were in the low-risk range of 0 to 1, 34.2% were in the intermediate risk range of 2 to 3, and 28.9% were in the high-risk category of 4 or greater(9). The mean score (± SD) of the modified NIH activity index was 6.47 ± 4.34 which is lower than the mean score of standard NIH activity index.
Correlation between morphologic lesions and parameter outcome:
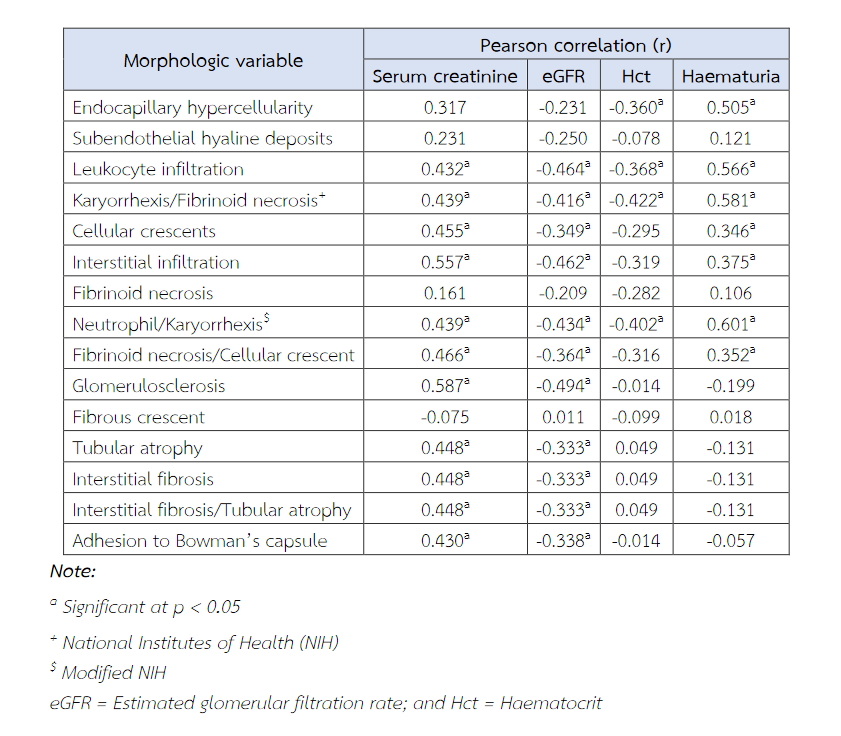
Leucocyte infiltration, karyorrhexis/fibrinoid necrosis, cellular crescents, interstitial infiltration, neutrophils infiltrate/karyorrhexis, fibrinoid necrosis/cellular crescent, glomerulosclerosis, tubular atrophy, interstitial fibrosis and adhesion to Bowman's capsule had correlation with both serum creatinine and eGFR (Table 6). In the activity index, serum creatinine/eGFR were strongest correlated with the interstitial infiltration variables (r = 0.557, p = 0.001) and fibrinoid necrosis/cellular crescent variables (r = 0.466, p = 0.003). In the chronicity index, correlations with serum creatinine/eGFR were strongest with the glomerulosclerosis variables (r = 0.587, p = 0.001) and interstitial fibrosis/tubular atrophy (r = 0.448, p = 0.005) (Table 6).
Table 3 Clinical and laboratory characteristics at the time of initial biopsy.
Haematuria showed parallel to the correlation of serum creatinine/eGFR with above morphologic lesions, hematuria correlated more closely with karyorrhexis/fibrinoid necrosis lesions (r = 0.581, p = 0.001) in parameters of NIH activity index as well as neutrophil/karyorrhexis lesions (r = 0.601, p = 0.001) in parameters of modified NIH activity index. Haematuria shows correlation with endocapillary hypercellularity (r = 0.505, p = 0.001).
Declined of haematocrit (Hct) significantly correlated with presence of endocapillary hypercellularity, leucocyte infiltration, karyorrhexis/fibrinoid necrosis and neutrophil/karyorrhexis. Haematocrit (Hct) showed no significant correlation with any morphologic variable in chronicity index.
Urine protein/creatinine ratio (UPCR) showed limited correlation with leucocyte infiltration (r = 0.399, p = 0.013). No significant correlation of serum albumin was found with any morphologic variable.
Correlations between parameter outcome and pathological indices:
The Pearson's correlations coefficient between renal outcome and pathological indices is shown in Table 7. Serum creatinine and eGFR were significantly correlated with all pathological indices. The most significant correlations were between serum creatinine and modified AI index (r = 0.461, p = 0.004) and between eGFR and modified AI index (r = -0.483, p = 0.002). Haematocrit (Hct) level and haematuria showed significant correlation with all indices, but no significant correlation was observed with chronicity index. In addition, there were no correlation between serum albumin/UPCR and any pathological indices (Table 7).
The eGFR was significantly decreased (less than 60 mL/min/1.73 m2), independently with these pathologic lesions, including presence of endocapillary hypercellularity ≥ 50% of total glomeruli, presence of subendothelial hyaline deposits ≥ 25% of total glomeruli, presence of fibrinoid necrosis/cellular crescent ≥ 25% of total glomeruli, presence of glomerulosclerosis ≥ 25% of total glomeruli, presence of fibrous crescent ≥ 5% of total glomeruli, interstitial fibrosis/tubular atrophy ≥ 10% of cortical area, and presence of adhesion to Bowman's capsule ≥ 25% of total glomeruli, respectively (Table 8).
Table 4 The frequency distribution of different classes of lupus nephritis according to International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification.
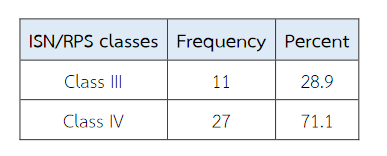
Table 5 NIH activity and chronicity indices with other indices.
Discussions
In our study, no significant correlation was identified between any morphologic variables and level of hematocrit. Austin et al. revealed that haematocrit less than 20% was a strong clinical predictor of poor prognosis(8) while there was no patient with haematocrit values less than 20% in our study that could affect the parameter outcome.
Fibrous adhesion of glomerular tuft to Bowman's capsule favours scarring from a previously active lesion rather than usual type segmental sclerosis(19). However, no attempts have been made to include the presence of adhesion to Bowman's capsule in the chronicity indices of lupus nephritis. We found that presence of adhesion to Bowman's capsule on renal biopsy was significantly correlated with high serum creatinine and decrease in eGFR, so it may provide useful prognostic information on renal survival in patients with lupus nephritis.
Our results revealed that the modified NIH indices show better correlations with clinical and outcome parameters than the standard NIH indices. We also observe significance difference in the mean of the NIH activity index scores and modified activity index scores (p < 0.001). In our study, the mean score (± SD) of modified NIH activity index was 6.47 ± 4.34 that lower than the mean score of standard NIH activity index. In our study, fibrinoid necrosis was present in 4 patients (10.5%) compare with 22 patients (57.9%) had fibrinoid necrosis and/or karyorrhexis that could affect these index scores.
Table 6 Correlations between serum creatinine and various morphologic variables: Pearson product-moment correlations.
For the activity index, we observed the presence of cellular/fibrocellular crescents and/or fibrinoid necrosis on renal biopsy was significantly correlated with several laboratory abnormalities, including high serum creatinine, decrease in eGFR, and increase microscopic haematuria. We found the presence of endocapillary hypercellularity was also associated with increase microscopic haematuria while there was no correlation between the presence of subendothelial deposits on renal biopsy with any laboratory outcome data. Similarly, the chronicity index had also shown correlation with the renal function. The presence of glomerulosclerosis, interstitial fibrosis and tubular atrophy, and presence of adhesion to Bowman's capsule on renal biopsy were significantly correlated with high serum creatinine and decrease in eGFR. There was no correlation between the presence of fibrous crescent on renal biopsy with any laboratory outcome data.
Furthermore, although modified NIH indices and standard NIH indices have been associated with renal outcome in LN, the interobserver reproducibility for the standard NIH activity and chronicity indices is relatively poor(18). Base of these findings, we suggest the presence of any of the histological features of the AI (endocapillary hypercellularity, cellular/fibrocellular crescent and/or necrosis) reportedly defines the patients at risk of developing renal failure. Similarly, the presence of any of the histological features of the CI (glomerulosclerosis, interstitial fibrosis and tubular atrophy) reportedly defines the patients at risk of developing renal failure.
The limitation of this study was the small sample size and this is a single centre study, further validation of these indices needs to be studied in larger center studies for their reproducibility.
Table 7 Pearson's correlations coefficient of various clinical outcome and pathologic parameters.
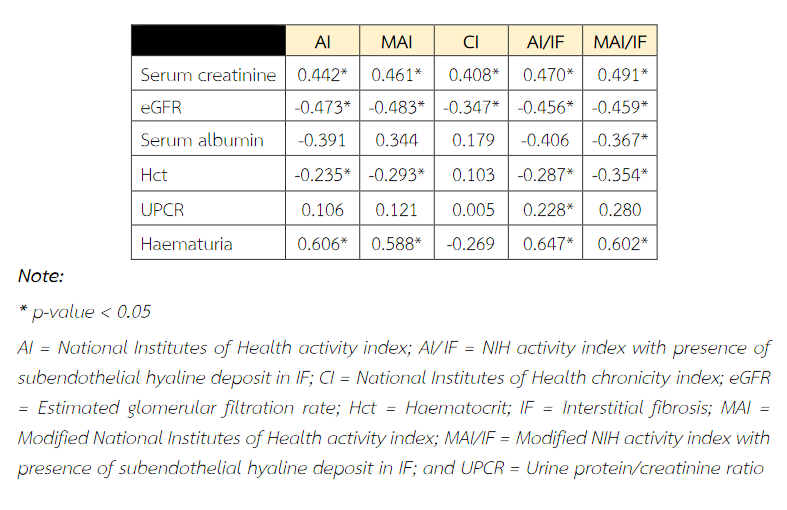
Table 8 Receiver operating characteristic curve for determining specific cut-off for percent of glomerular involvement in each parameter for estimated patient with GFR less than 60 mL/min/1.73 m2.

Conclusion
We suggest that the standard activity and chronicity indices in lupus nephritis could be improved by using individual morphologic variables that are easier to be performed in clinical practice for predicting renal outcome.
References
- Almaani S, Meara A, Rovin BH. Update on Lupus Nephritis. Clinical Journal of the American Society of Nephrology [Internet]. American Society of Nephrology (ASN); 2016 Nov 7;12(5):825–35. Available from: http://dx.doi.org/10.2215/cjn.05780616v.
- Sada K-E, Makino H. Usefulness of ISN/RPS Classification of Lupus Nephritis. Journal of Korean Medical Science [Internet]. Korean Academy of Medical Sciences (KAMJE); 2009;24(Suppl 1):S7. Available from: http://dx.doi.org/10.3346/jkms.2009.24.S1.S7.
- Satirapoj B, Tasanavipas P, Supasyndh O. Clinicopathological Correlation in Asian Patients with Biopsy-Proven Lupus Nephritis. International Journal of Nephrology [Internet]. Hindawi Limited; 2015;2015:1–6. Available from: http://dx.doi.org/10.1155/2015/857316.
- Grande JP, Balow JE. Renal biopsy in lupus nephritis. Lupus [Internet]. SAGE Publications; 1998 Nov;7(9):611–7. Available from: http://dx.doi.org/10.1191/096120398678920730.
- Nasri H, Ahmadi A, Baradaran A, et al. Clinicopathological correlations in lupus nephritis; a single center experience. J Nephropathol. 2014;3(3):115–120. doi:10.12860/jnp.2014.22.
- Bruijn JA. Lupus Glomerulonephritis. Fundamentals of Renal Pathology [Internet]. Springer New York; 79–98. Available from: http://dx.doi.org/10.1007/978-0-387-31127-2_8.
- Weening JJ, D'agati VD, Schwartz MM, Seshan SV, Alpers CE, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney International [Internet]. Elsevier BV; 2004 Feb;65(2):521–30. Available from: http://dx.doi.org/10.1111/j.1523-1755.2004.00443.x.
- Austin HA, Boumpas DT, Vaughan EM, Balow JE. Predicting renal outcomes in severe lupus nephritis: Contributions of clinical and histologic data. Kidney International [Internet]. Elsevier BV; 1994 Feb;45(2):544–50. Available from: http://dx.doi.org/10.1038/ki.1994.70.
- Austin HA, Muenz LR, Joyce KM, Antonovych TT, Balow JE. Diffuse proliferative lupus nephritis: Identification of specific pathologic features affecting renal outcome. Kidney International [Internet]. Elsevier BV; 1984 Apr;25(4):689–95. Available from: http://dx.doi.org/10.1038/ki.1984.75.
- Parikh SV, Alvarado A, Malvar A, Rovin BH. The Kidney Biopsy in Lupus Nephritis: Past, Present, and Future. Seminars in Nephrology [Internet]. Elsevier BV; 2015 Sep;35(5):465–77. Available from: http://dx.doi.org/10.1016/j.semnephrol.2015.08.008.
- Appel GB, Radhakrishnan J a. i., D'Agati VD. Secondary Glomerular Disease. Brenner and Rector's The Kidney [Internet]. Elsevier; 2011;1192–277. Available from: http://dx.doi.org/10.1016/b978-1-4160-6193-9.10032-6.
- Wernick RM. Reliability of Histologic Scoring for Lupus Nephritis: A Community-based Evaluation. Annals of Internal Medicine [Internet]. American College of Physicians; 1993 Oct 15;119(8):805. Available from: http://dx.doi.org/10.7326/0003-4819-119-8-199310150-00006.
- Grootscholten C, Bajema IM, Florquin S, Steenbergen EJ, Peutz-Kootstra CJ, Goldschmeding R, et al. Interobserver agreement of scoring of histopathological characteristics and classification of lupus nephritis. Nephrology Dialysis Transplantation [Internet]. Oxford University Press (OUP); 2007 Aug 17;23(1):223–30. Available from: http://dx.doi.org/10.1093/ndt/gfm555.
- Schwartz MM, Lan S, Bernstein J, Hill GS, Holley K, Lewis EJ. Irreproducibility of the Activity and Chronicity Indices Limits Their Utility in the Management of Lupus Nephritis. American Journal of Kidney Diseases [Internet]. Elsevier BV; 1993 Apr;21(4):374–7. Available from: http://dx.doi.org/10.1016/s0272-6386(12)80263-0.
- Hill GS, Delahousse M, Nochy D, Tomkiewicz E, Rémy P, Mignon F, et al. A new morphologic index for the evaluation of renal biopsies in lupus nephritis. Kidney International [Internet]. Elsevier BV; 2000 Sep;58(3):1160–73. Available from: http://dx.doi.org/10.1046/j.1523-1755.2000.00272.x.
- Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney International [Internet]. Elsevier BV; 2018 Apr;93(4):789–96. Available from: http://dx.doi.org/10.1016/j.kint.2017.11.023.
- Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism [Internet]. Wiley; 1997 Sep;40(9):1725–1725. Available from: http://dx.doi.org/10.1002/art.1780400928.
- Dasari S, Chakraborty A, Truong L, Mohan C. A Systematic Review of Interpathologist Agreement in Histologic Classification of Lupus Nephritis. Kidney International Reports [Internet]. Elsevier BV; 2019 Oct;4(10):1420–5. Available from: http://dx.doi.org/10.1016/j.ekir.2019.06.011.
- Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Membranous Lupus Nephritis, ISN/RPS Class V. American Journal of Kidney Diseases [Internet]. Elsevier BV; 2017 Aug;70(2):e13–e15. Available from: http://dx.doi.org/10.1053/j.ajkd.2017.06.003.


