Comparison between unbuffered and buffered formalin fixatives for oestrogen receptor and Ki67 immunostains in breast cancer specimens: a pilot study
Chetsada Chunkaruhart1, Nopporn Tipparawong2
and Kroonpong Iampenkhae2*
1. Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
2. Department of Pathology, King Chulalongkorn Memorial Hospital, Bangkok, Thailand
Correspondence to: Dr Kroonpong Iampenkhae, Department of Pathology, Floor 13, His Majesty King Ananda Mahidol Building, 1873 King Chulalongkorn Memorial Hospital, Rama IV Road, Pathumwan, Bangkok 10330 Thailand. Telephone: +66 (0) 2 256 4235, +66 (0) 2 256 4581 Facsimile: +66 (0) 2 652 4208 Email: kroonpong@gmail.com
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
|
Submitted: |
12 April 2021 |
|
Accepted: |
21 April 2021 |
|
Published: |
1 October 2021 |
Abstract
Pre-analytic steps are important for the immunohistochemical study as in the case of breast cancers. The initial process is the fixation in which the 10% neutral buffered formalin (NBF) is recommended. 10% formalin is less expensive and easier to prepare but its effect on the immunostaining is controversial. The aim of this study was to compare the effect of 10% formalin with 10% NBF on oestrogen receptor (ER) and Ki67 immunostains in breast cancer specimens. Thirty breast cancer specimens were collected. Each tumour was bisected and separately fixed in 10% formalin and 10% NBF, processed, and immunostained with ER and Ki67. Whole slides were scanned and evaluated by digital image analysis. A semiquantitative H-score was determined. No significant differences, both the percentage and H-score, of the ER- and Ki67-positive cells were found between breast cancer specimens fixed in 10% formalin and 10% NBF. In conclusion, 10% formalin could be used as a fixative for immunohistochemical study for breast biomarkers. The unbuffered fixative is less expensive and easier to prepare, compared to 10% neutral buffered formalin. Additional study is reasonable to evaluate the effect of 10% formalin to the quality of nucleic acid.
Keywords: 10% formalin; 10% neutral buffered formalin; breast cancers; ER; Ki67
Introduction
Breast cancer is one of the leading cancers in Thailand, with the highest incidence among cancers in women(1-3). During 2013 – 2015, the age-standardised incidence rate (ASR) of breast cancer was 31.4 per 100,000 in Thai women(1). Like many other malignancies, breast cancer is not a single disease as it can be categorised into luminal A, luminal B, HER2/Neu-overexpression, and basal-like subtypes(4). College of American Pathologists (CAP)/American Society of Clinical Oncology (ASCO) guidelines recommend oestrogen receptor (ER), progesterone receptor (PR) and HER2/neu (HER2) analysis for all new diagnoses of breast cancer. In routine practice, all breast cancer specimens in Thailand are subject to immunohistochemical study for evaluation of ER, PR, HER2 and Ki67. These biomarkers have long been used to select an appropriate treatment for an individual patient(4,5). ER and PR are the prognostic factors and predictive for the benefit with the endocrine treatment(6).
Pre-analytic process is especially important to ensure the good immunohistochemistry result, and fixation is one of the crucial steps. While 10% Neutral buffered formalin (NBF) is recommended(7-9), some laboratories in Thailand - a middle-income country - still use 10% formalin as it is much less expensive. In addition, 10% formalin is easily prepared by only adding nine parts of water, preferably distilled, to one part of the stock formalin. This is in contrast with the preparation of 10% NBF that needs buffer, typically sodium phosphate, to adjust the pH(10).
Although 10% formalin and 10% NBF have been compared as fixatives; for example, Arima et al. found Ki67 was significantly higher when 10% NBF was used(11), the Ki67 was scored by manual counting in the hot spots without the use of digital image analysis (DIA). The purpose of our study was to re-evaluate the effect of 10% formalin on breast cancer biomarkers, using ER and Ki67 as a model, and compare the results with those obtained from 10% NBF fixative. DIA was used for the objective scoring. This study was approved by the Institutional Review Board at the Faculty of Medicine, Chulalongkorn University (IRB # 132/64).
Materials and Methods
Fixatives:
10% formalin was prepared weekly by dissolving 100 mL of commercially available formalin (100% formalin, TOA Dovechem Industries Co.,Ltd., Bangkok, Thailand) in 900 mL of distilled water. 10 % formalin was prepared fresh from stock solutions before use every week. 10% NBF (Bio-Optica Milano S.p.A., Milan, Italy) was purchased in a ready-to-use form. The pH level of 10% formalin and 10% NBF was 5 and 7, respectively (measured by Litmus paper). All fixatives were precautionarily stored and used at room temperature.
Specimen collection and fixation:
Thirty fresh specimens of the breast cancer were received from the Department of Surgery, King Chulalongkorn Hospital. Cold ischaemia time is less than one hour. For each case, the tumour was cut into two pieces (less than 5 mm in thickness). One half was immediately fixed in 10% formalin, and the other half in 10% NBF for 24 – 48 hours. The 1:15 ratio of tissue volume to formalin volume was used. After fixation, both halves of the tumour were routinely processed and embedded in the same paraffin block.
Immunohistochemistry:
Four-µm-thick sections were deparaffinization and underwent antigen retrieval using EnVision FLEX High pH Target Retrieval Solution for Dako PT LINK tank (Agilent Technologies, California, United States of America). The antibodies used in the study were also from Dako, including a monoclonal rabbit Anti-Human Estrogen Receptor alpha antigen (clone EP1, ready-to-use) and a monoclonal mouse Anti-Human Ki67 antigen (clone MIB-1, ready-to-use). All immunostains were carried out with a Dako Autostainer Link 48.
Image and data analyses:
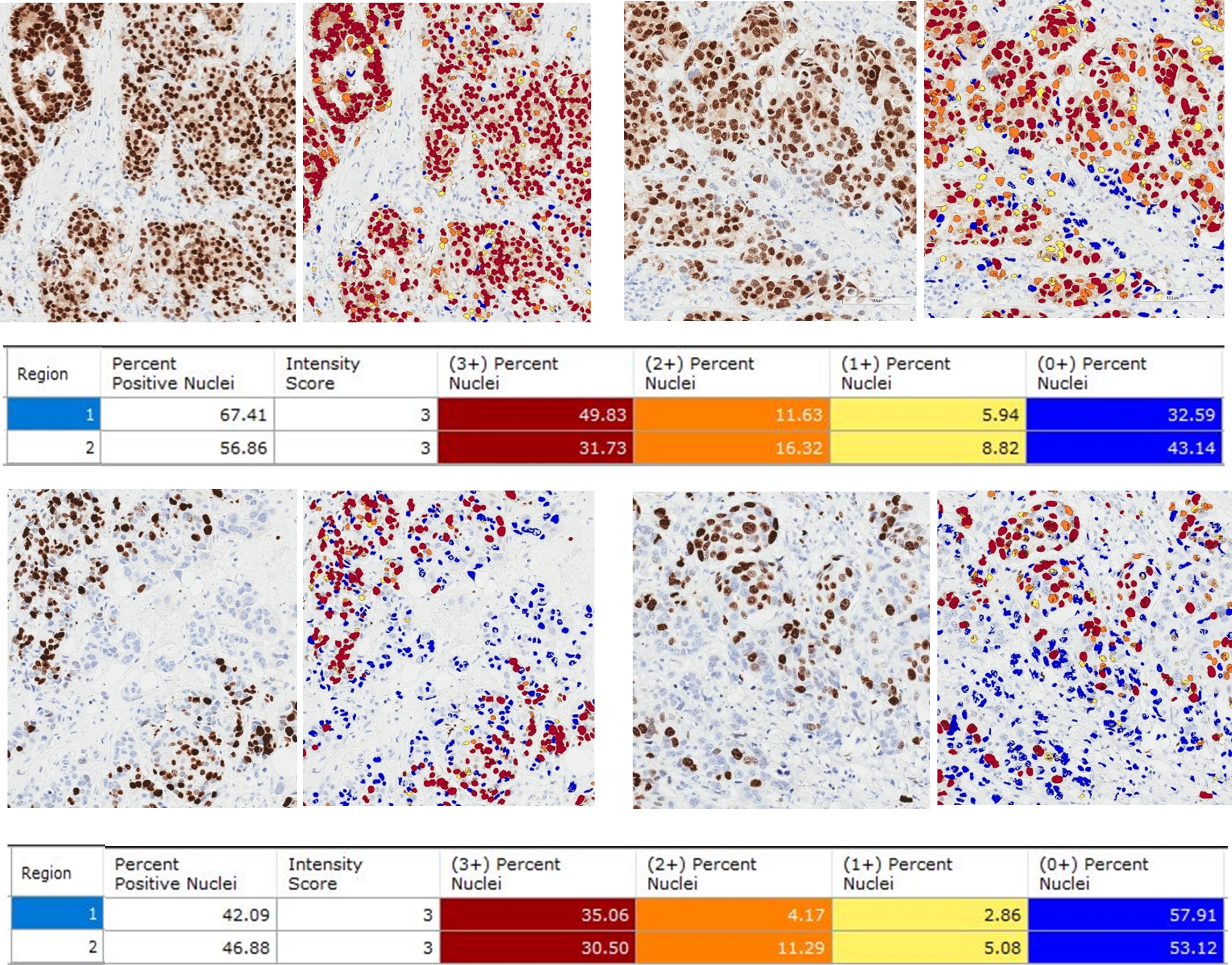
Whole ER- and Ki67-stained sections were converted into digital format using Aperio CS2 scanner (Aperio Technologies Inc., California, United States of America) using Aperio ImageScope (v12.3.2.5030). The tumour fixed with 10% NBF and 10% formalin in each whole section was selected for annotation. ER- and Ki67-positive nuclei were analysed by Nuclear v9 algorithm (Aperio's Image Analysis Kit) on whole tumour areas. Parameters examined included percentage of positive cells and percentage of different staining intensity (0, 1+, 2+, 3+) (Figure 1).
A semiquantitative approach was also performed to assign an H-score [H-score (0-300 scale) = 3 x (% at 3+) + 2 x (% at 2+) + 1 x (% at 1+)]. Results of ER and Ki67 were compared between specimens with different fixatives, using the Mann–Whitney U test (IBM SPSS version 22.0, SPSS Inc., Chicago, IL). The p-value of < 0.05 was considered statistical significance.
Figure 1 Example of digital image analysis of ER (Upper panel) and Ki67 (Lower panel) immunostains in breast cancer tissue, with prior 10% formalin (Left) and 10% neutral buffered formalin (Right) fixation (Nuclear v9 algorithm, Aperio Image Analysis software).
Results
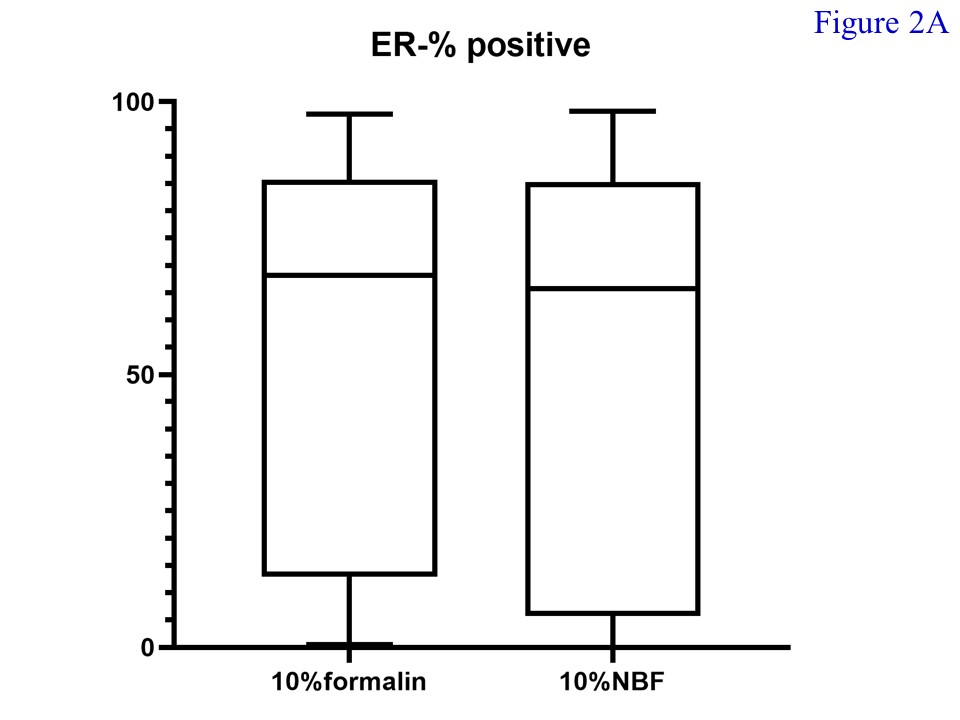
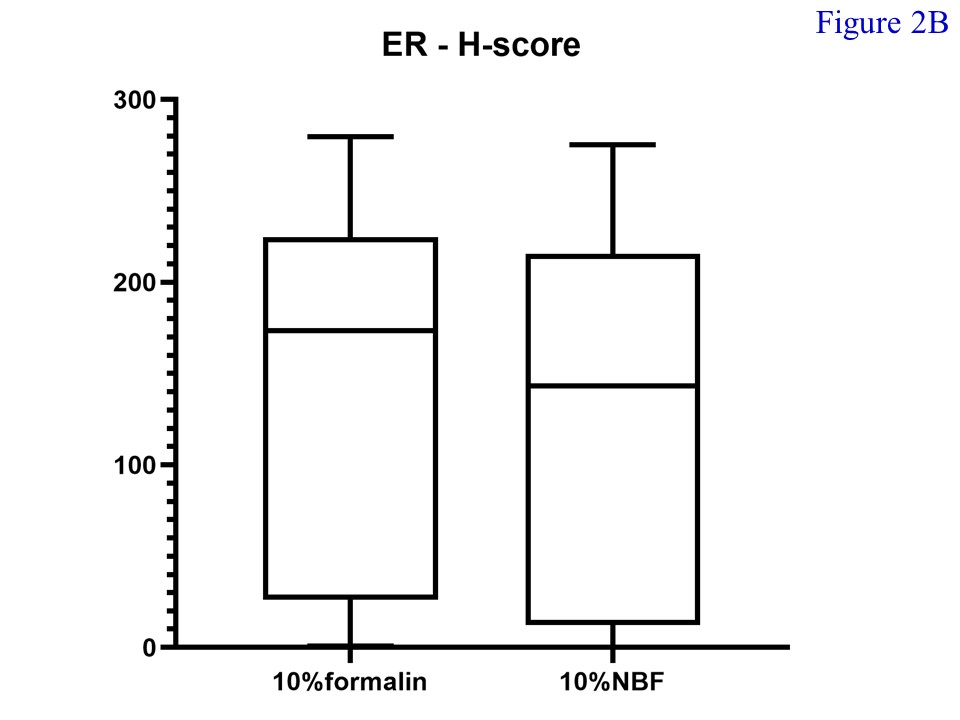
Of the total 30 cases of female breast cancers enrolled for the study, 29 were invasive ductal carcinoma while the remaining was an invasive lobular carcinoma. The mean age of patients was 57.2 years. The median percentage of ER-positive cells in tissue fixed with 10% formalin (68.18; min – max = 0.49 – 97.62; mean = 52.78) and 10% NBF (65.75; min – max = 0.04 – 98.16; mean = 52.64) was not significantly different (Figure 2A) (p = 0.848). Also, the median H-score of ER-positive cells in tissue fixed with 10% formalin (173.55; min – max = 0.70 – 279.75; mean = 131.10) and 10% NBF (143.30; min – max = 0.06 – 275.23; mean = 127.99) did not significantly differ (p = 0.734) (Figure 2B).
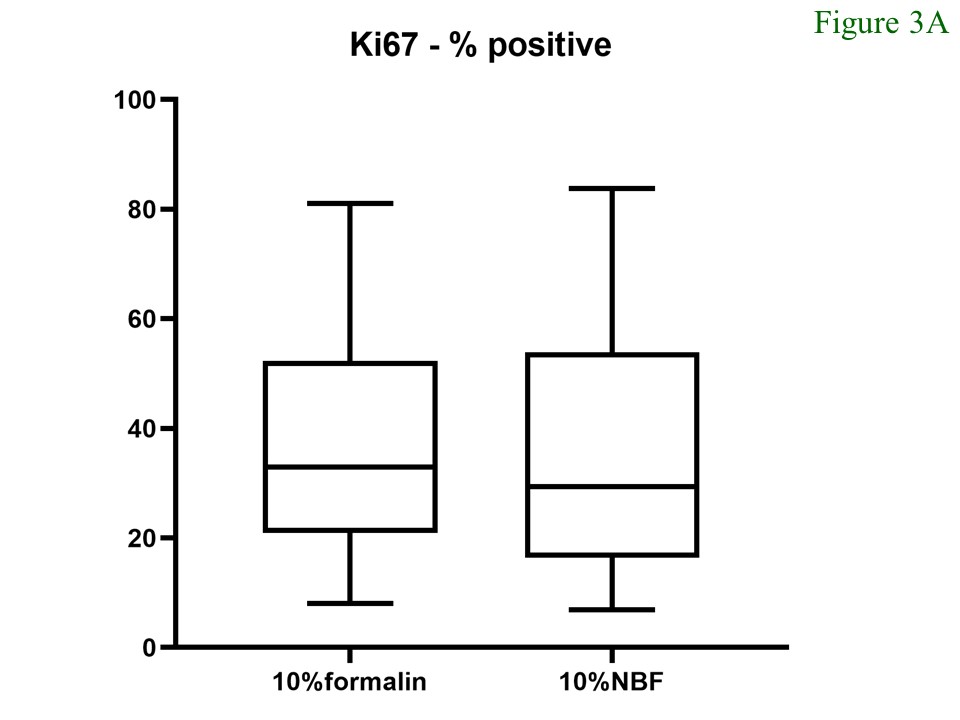
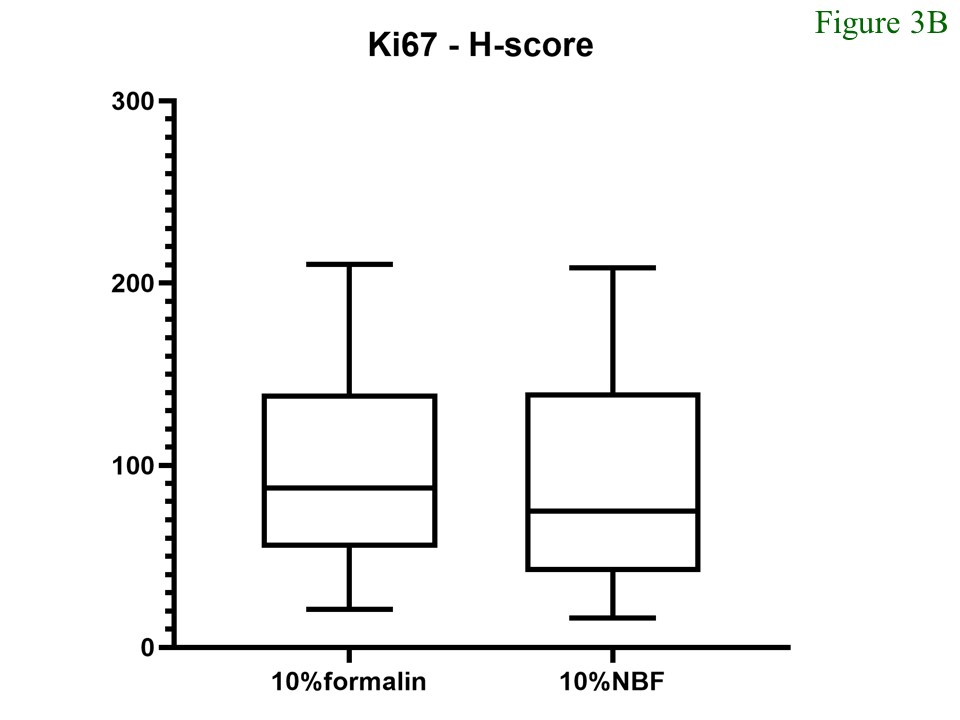
For the Ki67 staining, the medians of Ki67 index of the tumours fixed in 10% formalin and 10% NBF were 32.94 (min – max = 7.99 – 81.06; mean = 37.29) and 29.45 (min – max = 6.87 – 83.78; mean = 35.39), respectively. The median H-score of ER-positive cells in tissue fixed with 10% formalin was 87.42 (min – max = 21.07 – 210.36; mean = 95.07) whereas that of the tissue fixed with 10% NBF was 75.0 (min – max = 16.25 – 208.38; mean = 89.29). Comparison of both parameters between the different fixatives did not show significant differences (Figure 3).
Figure 2 Percentages (A) and H-Score (B) of ER-positive cells. There was no significant difference noted between samples fixed with 10% formalin and 10% neutral buffered formalin (p = 0.848 and 0.734, respectively).
Figure 3 Percentages (A) and H-Score (B) of Ki67-positive cells. There was no significant difference noted between samples fixed with 10% formalin and 10% neutral buffered formalin (p = 0.478 and 0.525, respectively).
Discussion
Evaluation of breast biomarkers is essentially important to select an appropriate treatment for the patients. Although the immunohistochemical method has remarkably been improved, with the use of automated immunostainers, pre-analytic steps such as cold ischaemic time (time to fixation), fixative (solution that prevents autolysis), fixation time (duration of fixation), are still vital to ensure the reliable results. Improper pre-analytic steps can significantly affect treatment decisions(12). These pre-analytic factors have been studied quite extensively in breast cancer biomarkers(13-19). The recommended cold ischaemic time and fixation time are as soon as possible (or less than one hour) and 6 – 72 hours, respectively, according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) 2020(7,24).
Formalin is a universal fixative in surgical pathology, and the volume of fixative should be sufficient or 20 times the volume of tissue(7,8). 10% buffered neutral formalin (BNF), pH (7.2 – 7.4), is recommended since both acidity or alkalinity can interfere with the fixation process(7,20). Compared to 10% NBF (130.80 Bahts/litre), 10% formalin (2.75 Bahts/litre) is much less expensive and easier to prepare but the latter is usually acid (pH = 5.0 to 5.5). Because of the cost and preparation process, 10% formalin is still used in some pathology laboratories. Differences between 10% NBF and 10% formalin on the performance of immunostaining are somewhat controversial. Unbuffered formalin was said to be a better fixative for the immunorecognition of many antigens(21).
Matsuda et al. studied formalin-fixed paraffin-embedded tissue of nude mice implanted with human uterine cervical cancer cells using immunostains and image analysis on limited microscopic fields(22). Compared to specimens fixed with 10% NBF, Ki-67 stain performed in samples with unbuffered formalin showed slightly higher expression. However, no statistical analysis was conducted in this study. Without image analysis, Burns JA et al. noted slightly higher staining intensities of HER2 in cancer tissue fixed with 10% NBF, compared to those prior fixed with unbuffered formalin although this did not reach the statistical significance(23).
The methods used for counting (manual vs. digital image analysis) may have accounted for the discrepancy. Digital image analysis (DIA) has been shown to be superior alternative to the manual biomarker scoring(24). Stålhammar et al. demonstrated that the DIA of Ki67 in hot spots was better than manual Ki67 counts in breast cancers(25). In our study of ER and Ki67 scoring using DIA, we were not able to show significant differences between breast cancer specimens fixed with 10% NBF and 10% formalin.
It should be noted that prolonged storage of unbuffered formalin may induce precipitation of white para-formaldehyde deposits and produce turbidity. Traces of formic acid are formed by oxidation, which decreases the quality of nuclear staining and leaches out haemosiderin resulting in formation of brown-black pigment called formalin pigment(26). To avoid prolonged storage of formalin, 10% formalin solution should be regularly prepared (e.g., weakly). NBF has a longer shelf life(23) and the tissue fixed with NBF has been shown to have better RNA quality(27,28). In our study, 10% formalin was freshly prepared every week. 10% NBF was shortly stored too. This is the possible explanation that 10% formalin seemed to be consistently superior to 10% NBF through not statically different.
PR and HER2 stains were not included in our study, further studies of these stains are considered. Whole tumour areas were analysed but more sample sizes may show the difference of the results. The aim of this study is the comparison of preserved antigenicity between unbuffered and buffered formalin, dividing the group of high and low ER and Ki67 expression may not be significant differences between groups. Since the molecular testing is increasingly needed, further studies are warranted to determine the effect of 10% formalin on the quality of nucleic acid.
Conclusion
10% formalin could be used as a fixative for immunohistochemical study for breast biomarkers. The unbuffered fixative is less expensive and easier to prepare, compared to 10% neutral buffered formalin. Additional study is reasonable to evaluate the effect of 10% formalin to the quality of nucleic acid.
References
- Imsamran W, Adisia P, Pongsatorn S, et al. Cancer in Thailand. IX., 2013 – 2015 Bangkok:National Cancer Institute 2015:6.
- Imsamran W, Chaiwerawattana A, Wiangnon S, et al. Cancer in Thailand. VIII., 2010 – 2012. Bangkok:National Cancer Institute 2015:12.
- Khuhaprema T, Attasara P, Sriplung H, et al. Cancer in Thailand. Vol. VII, 2007 – 2009. Bangkok: National Cancer Institute 2013:10.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ. Panel members: personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Annals of Oncology 2013;24:2206–2223.
- Atif Ali Hashmi et. al. Ki67 index in intrinsic breast cancer subtypes and its association with prognostic parameters. BMC Research Notes 2019;12(1).
- Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: past, present and future. Seminars in Cancer Biology 2018;52(Pt 1):56-73.
- Kimberly H. Allison, M. Elizabeth H. Hammond, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. Journal of Clinical Oncology 2020; 38:1346-1366.
- Wolff AC, Hammond MEH, Allison KH, er al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Archives of Pathology & Laboratory Medicine 2018;142(11):1364-1382.
- Nielsen, T. O., Leung, S. C. Y., Rimm, D. L., et. al. Assessment of Ki67 in Breast Cancer: Updated recommendations from the International Ki67 in Breast Cancer Working Group. JNCI: Journal of the National Cancer Institute 2020;djaa201.
- Dey, P. Basic and Advanced Laboratory Techniques in Histopathology and Cytology (1st ed. 2018 ed.). Springer. 2018:10-11.
- Arima N, Nishimura R, Osako T, et al. The importance of tissue handling of surgically removed breast cancer for an accurate assessment of the Ki-67 index. Journal of Clinical Pathology 2016;69:255–259.
- Maung R, Work pattern of Canadian pathologists. Diagnostic Histopathology 2016;22:288–293.
- Portier BP, Wang Z, Downs-Kelly E, et al. Delay to formalin fixation 'cold ischemia time': effect on ERBB2 detection by in-situ hybridization and immunohistochemistry. Modern Pathology 2013;26:1–9.
- Khoury T, Sait S, Hwang H, et al. Delay to formalin fixation effect on breast biomarkers. Modern Pathology 2009;22:1457–67.
- Walker RA. Immunohistochemical markers as predictive tools for breast cancer. Journal of Clinical Pathology 2008;61:689–96.
- Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology. Archives of Pathology & Laboratory Medicine 2008;132:1929–35.
- Goldstein NS, Ferkowicz M, Odish E, et al. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. American Journal of Clinical Pathology 2003;120:86–92.
- Goldstein NS, Hewitt SM, Taylor CR, et al. Members of Ad-Hoc committee on immunohistochemistry standardization. Recommendations for improved standardization of immunohistochemistry. Applied Immunohistochemistry & Molecular Morphology 2007;15:124–33.
- Tong LC, Nelson N, Tsourigiannis J, et al. The effect of prolonged fixation on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast cancer: a prospective study. The American Journal of Surgical Pathology 2011;35:545–52.
- Thavarajah, R., Mudimbaimannar, V. K., Rao, U. K., Ranganathan, K., & Elizabeth, J. Chemical and physical basics of routine formaldehyde fixation. Journal of Oral and Maxillofacial Pathology 2012;16(3):400.
- Arnold, M. M., Srivastava, S., Fredenburgh, J., et al. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotechnic and Histochemistry 1996;71:224–230.
- Matsuda, Y., Fujii, T., Suzuki, T., Yamahatsu, K., Kawahara, K., Teduka, K., Kawamoto, Y., Yamamoto, T., Ishiwata, T., & Naito, Z. Comparison of fixation methods for preservation of morphology, RNAs, and proteins from paraffin-embedded human cancer cell-implanted mouse models. Journal of Histochemistry & Cytochemistry 2011;59(1): 68–75.
- Burns, J. A., Li, Y., Cheney, C. A., Ou, Y., Franlin-Pfeifer, L. L., Kuklin, N., & Zhang, Z.-Q. (2008). Choice of fixative is crucial to successful immunohistochemical detection of phosphoproteins in paraffin-embedded tumor tissues. Journal of Histochemistry & Cytochemistry 2008;57(3):257–264.
- Stålhammar, G., Fuentes Martinez, N., Lippert, M., Tobin, N. P., Mølholm, I., Kis, L., Rosin, G., Rantalainen, M., Pedersen, L., Bergh, J., Grunkin, M., & Hartman, J. Digital image analysis outperforms manual biomarker assessment in breast cancer. Modern Pathology 2016.
- Stålhammar, G., Robertson, S., Wedlund, L., Lippert, M., Rantalainen, M., Bergh, J., & Hartman, J. Digital image analysis of Ki67 in hot spots is superior to both manual Ki67 and mitotic counts in breast cancer. Histopathology 2018 May;72(6):974-989.
- Shariff, S., & Kaler, A. K.. Principles and Interpretation of Laboratory Practices in Surgical Pathology (1st ed.). Jaypee Brothers Medical Publishers Pvt Ltd. 2016:5-7.
- Chung, J.-Y., Braunschweig, T., Williams, R., Guerrero, N., Hoffmann, K. M., Kwon, M., Song, Y. K., Libutti, S. K., & Hewitt, S. M. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. Journal of Histochemistry & Cytochemistry 2008;56(11):1033–1042.
- Seo, A. N., Kim, J.-H., Lee, D., Jeong, J. Y., & Park, J.-Y. Comparison of the DNA preservation in neutral-buffered formalin-fixed, paraffin-embedded tissue and in non-buffered formalin-fixed, paraffin-embedded tissue. The Korean Journal of Pathology, 2011;45(6): 549.