Loop-mediated isothermal amplification for detection of Mycobacterium tuberculosis and evaluation in paraffin-embedded lymph nodes
Tanin Titipungul M.D., Emorn Panomsri M.Sc., Sakkarn Sangkhamanon M.D.,
Chawalit Pairojkul M.D., Yaovalux Chamgramol Ph.D.
Department of Pathology, Faculty of Medicine,
Khon Kaen University, Khon Kaen, Thailand
Correspondence: Yaovalux Chamgramol, Ph.D.
Department of Pathology, Faculty of Medicine,
Khon Kaen University, Khon Kaen, 40002, Thailand.
Tel. +66-043-363691, Fax. +66-043-348388,
E-mail: cyaova@yahoo.com
ABSTRACT
Background: Tuberculosis is a treatable disease, but remains a major cause of mortality. Culture for Mycobacterium is needed to make a definitive diagnosis; thus, loop-mediated isothermal amplification (LAMP) was attempted for its detection in formalin-fixed, paraffin-embedded (FFPE) samples.
Materials and method: Archival FFPE blocks were used from 64 patients with a clinical diagnosis of tuberculous lymphadenitis from Srinagarind Hospital, Khon Kaen University, between 2007 and 2011. The clinical data, routine surgical pathologic examination, AFB stain, culture of Mycobacterium were researched retrospectively. Six primers were used to target the M. tuberculosis 16S rRNA gene for specific amplification and hydroxy naphthol blue (HNB) to detect the LAMP products.
Results: Seven of 23 (30%) samples of tuberculous lymphadenitis (culture positive for M. tuberculosis) were LAMP positive while all respective 23 and 21 cases of reactive hyperplasia lymph node (culture negative for Mycobacterium) and non-tuberculous lymphadenitis (culture positive for others Mycobacterium, excluding M. tuberculosis) were LAMP negative. Compared to culture, the sensitivity of LAMP in detecting M. tuberculosis in FFPE lymph node was 30.4%, specificity 100%, positive predictive value 100%, and negative predictive value 71.9%.
Conclusion: LAMP for diagnosis of tuberculous lymphadenitis in FFPE lymph nodes has limitations because of the degradation of M. tuberculosis DNA in paraffin-embedded samples. Its simplicity, quick-application, high specificity and low cost make it an alternative in fresh clinical samples. Further studies on fresh lymph nodes are warranted.
Keywords: Loop-mediated isothermal amplification, LAMP, Mycobacterium tuberculosis, tuberculous lymphadenitis
INTRODUCTION
Tuberculosis (TB) is a common chronic infectious disease caused by various strains of mycobacteria (Mycobacterium tuberculosis complex strains, e.g. M. tuberculosis, M. microti, M. bovis, M. africanum). M. tuberculosis is the most widespread infectious disease among humans [1]. TB causes an estimated 1.7 million deaths each year with more than 9 million new cases reported worldwide [2]. Typically, infection with M. tuberculosis manifests as pulmonary TB, but it can also affect other parts of the body in up to one-third of cases. Tuberculous lymphadenitis is the most common presentation of extra-pulmonary TB, particularly among HIV-infected patients [3].
A definitive diagnosis of TB is made by identifying Mycobacterium in a clinical samples; however, the difficult and time-consuming culture means that treatment is begun before cultures are confirmed [4]. Routine pathological diagnosis of tuberculous lymphadenitis depends on histopa-thology and detection of acid-fast bacilli using the Ziehl-Neelson stain (AFB stain). Among 89 (67 HIV-positive) tuberculous lymphadenitis-proven patients, histology and culture of a lymph node biopsy had the highest diagnostic yield (85% and 88%, respectively), followed by detection of acid-fast bacilli in biopsy smear (53%) [5]. A study among US immigrant patients (with culture positive tuberculous lymphadenitis samples) showed positive AFB staining in 44% (15/34 cases) [6].
In 2000, a novel technique that rapidly amplifies target DNA under isothermal conditions —the loop-mediated isothermal amplification (LAMP) method—was developed by Notomi [7]. LAMP employs a DNA polymerase with strand-displacement activity, along with two inner primers (FIP, BIP), outer primers (F3, B3) and loop primers (LF, LB), which recognize six separate regions within the target DNA [7]. The LAMP assay has a high specificity for Mycobacterium infection in fresh clinical samples [8-12]; however, no study of LAMP for M. tuberculosis of lymph node in FFPE samples has been conducted. We therefore planned a retrospective investigation of the potential use of LAMP as a simple assay for detection of M. tuberculosis using FFPE lymph nodes for pathological diagnosis.
MATERALS AND METHODS
Patients and tissue samples
Sixty-four patients who underwent lym-phadenectomy and tissue culture for diagnosis of tuberculous lymphadenitis at Srinagarind Hospital (Khon Kaen University) between 2007 and 2011 were included. This retrospective study was approved by the Ethics Research Committee, Faculty of Medicine, Khon Kaen University (HE531403).
Archival FFPE lymph node blocks were obtained from the Pathology Department. Patient information (including laboratory results) was obtained from medical records. Detailed clinico-pathological data are summarized in Table 1. Sixty-four lymph node specimens were divided into three groups, according to the results of culture for Mycobacterium: 1) the reactive hyperplasia lymph node (RHL) group had a negative culture for Mycobacterium (n=20), 2) the tuberculosis lymphadenitis (TBL) group had a positive culture for M. tuberculosis (n=23), and 3) the non-tuberculous lymphadenitis (NTL) group had a positive culture for Mycobacterium spp. but not for M. tuberculosis (n=21).
DNA extraction
Genomic DNA was extracted and purified from FFPE tissues using a QIAamp® DNA FFPE tissue kit (Qiagen, Germany). Ten-micrometer-thick paraffin sections were deparaffinized in xylene 2 times and dehydrated twice with alcohol. These sections were rehydrated with alcohol 3 times. Tissue sections were scraped into a microcentrifuge tube and lysed under denaturing conditions with proteinase K at 56°C for 1 hr. The lysate was applied to the QIAamp MinElute column and the bound DNA eluted with 50 ^L of ATE buffer. The DNA concentration and its purity were determined using a NanoVue™ spectrophotometer (GE healthcare, NJ, USA).
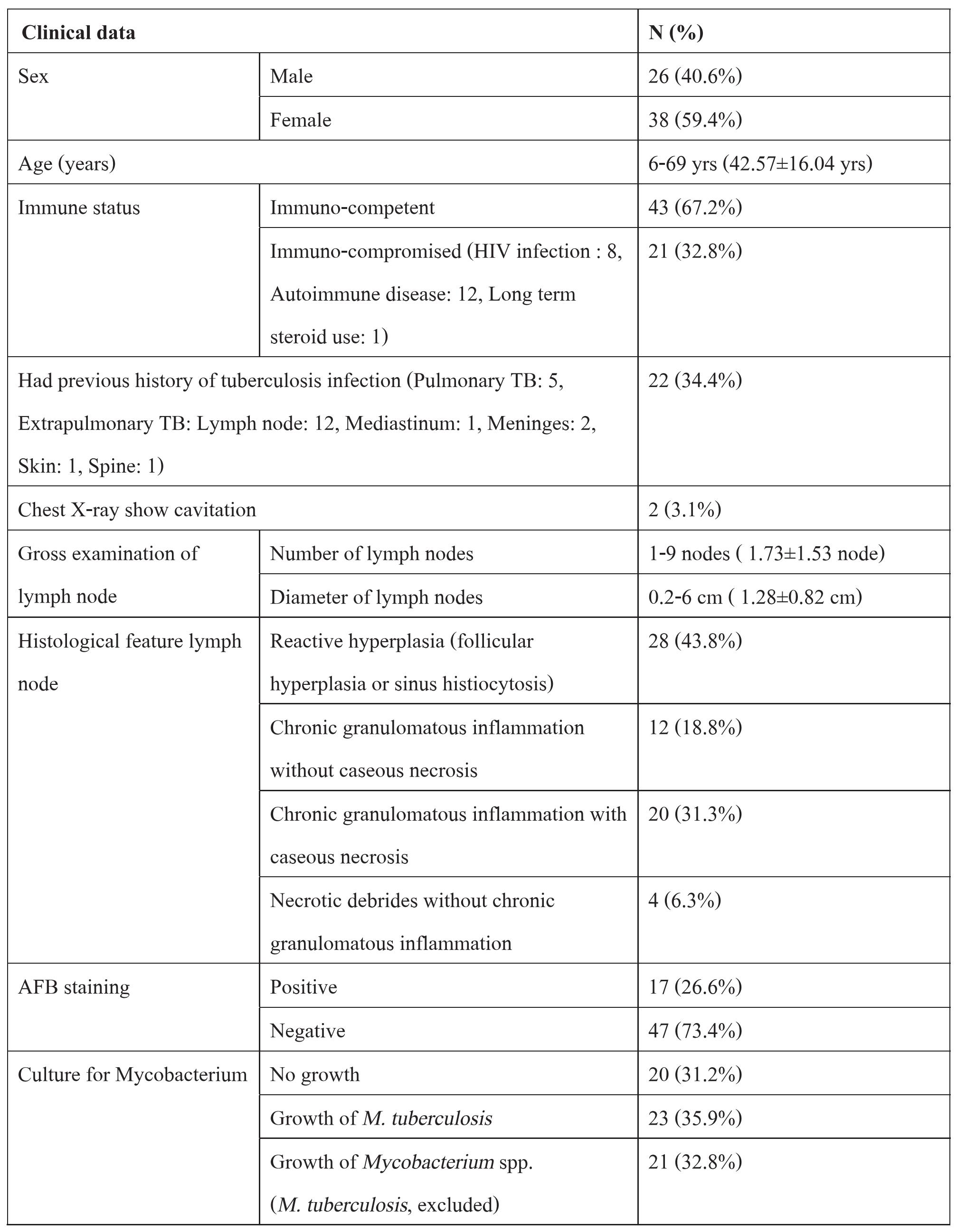
Table 1 Climco-pathologicalfeaturesof the 64samplesusedforLAMP assay
LAMP assay
Six primers recognizing distinct regions of the 16S rRNA gene of M. tuberculosis (Figure 1) were designed for specific amplification using the PrimerExplorer V3 software (Eiken Chemical; https://primerexplorer.jp/lamp3.0.0/index.html). A primer set was composed of outer primers F3 and B3, inner primers FIP and BIP, and loop primers FLP and BLP. LAMP reactions were performed in a 25-^L reaction mixture [13]; comprising: 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 8 mM MgSO4, Tween 20 (0.1%), 1.6 M betaine, 1.4 mM deoxynucleoside triphosphate, 1.6 ^mol each of the inner primers FIP and BIP, 0.2 ^mol each of the outer primers F3 and B3, 0.8 ^mol each of the loop primers FLP and BLP and 8 U Bst DNA polymerase (New England Biolabs). Different volumes of template DNA were tested but 7.5 ^L DNA solution extracted was optimal. Double-distilled water was finally used to make up the volume to 25 ^L. No template DNA was added in the negative control reaction. The mixture was incubated at 65°C in a heat block for 60 min then at 80°C for 5 min to terminate the reaction.
Detection of LAMP product
A LAMP positive reaction was determined by visual assessment of the amplification reaction indicated by the hydroxy naphthol blue (HNB) changing color from violet to sky blue [7,13] (Figure 2).
Statistical analysis
The statistical evaluation was performed using SPSSTM 17 (KKU license). The number and diameter in the LAMP positive and LAMP negative groups were compared using the T-Test. The other data were analysed using the Chi-square test. Statistical significance was set at p <0.05.

Figure 1 The 6 primers recognizing distinct regions of the 16S rRNA gene of M. tuberculosis. A primer set comprised the outer primers (F3 and B3), the inner primers (FIP and BIP) and the loop primers (FLP and BLP).
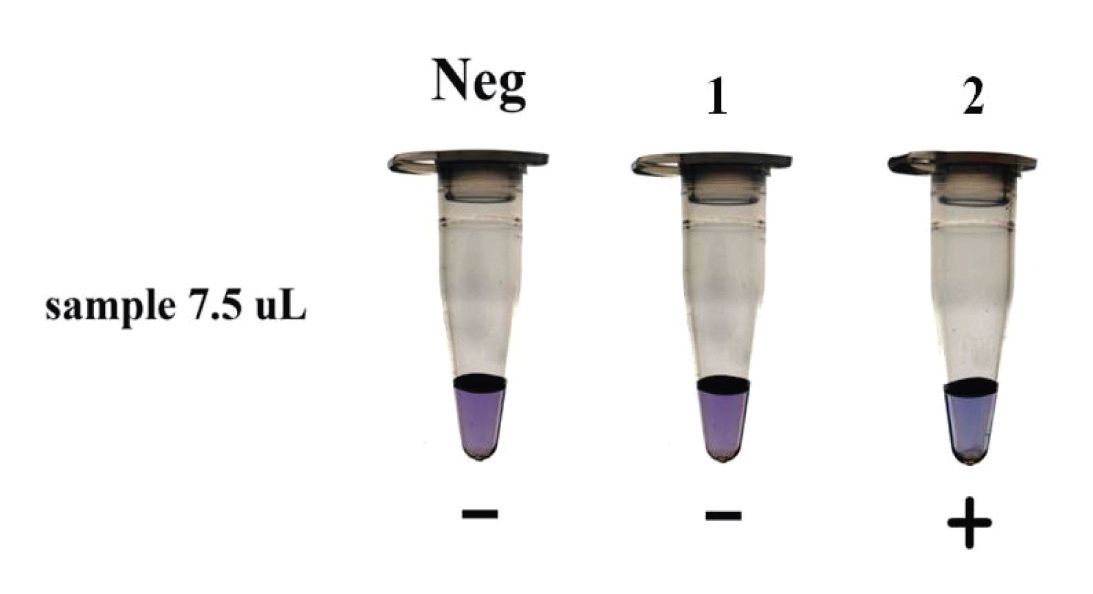
Figure 2 Visual examination of LAMP products (amplification amount of target DNA) by HNB, Neg (negative control); - (negative reaction, violet); + (positive reaction, sky blue); 1 and 2 (patient 1 and patient 2).
RESULTS
All FFPE samples yielded DNA extraction with different concentrations as determined by a spectrophotometer at O.D. 260 and tested with agarose gel (data not shown).
In the TBL group (n=23), 7 samples were LAMP-/AFB+ while 1 was LAMP+/AFB- and 9 were LAMP-/AFB- (Table 2). Seven of the TBL samples (7/23, 30.43%) were LAMP+ while all of the RHL group (0/20) and NTL group were LAMP. Comparing these results to the cultures positive for M. tuberculosis, the respective sensitivity and specificity of LAMP was 30.4% and 100%. The respective positive (PPV) and negative (NPV) predictive values were 100% and 71.9% (p-value <0.001). Note that our design was only for LAMP detection of
M. tuberculosis, so the sensitivity and specificity for a NTL group was not calculated. The AFB was negative in all of the cases of the RHL group (0/20) but positive in four of the cases of NTL group (4/21) and 13 of the TBL group (13/23). AFB had a sensitivity of 56.5%, a specificity of 90.2%, a PPV of 76.5%, and a NPV of 78.7%.
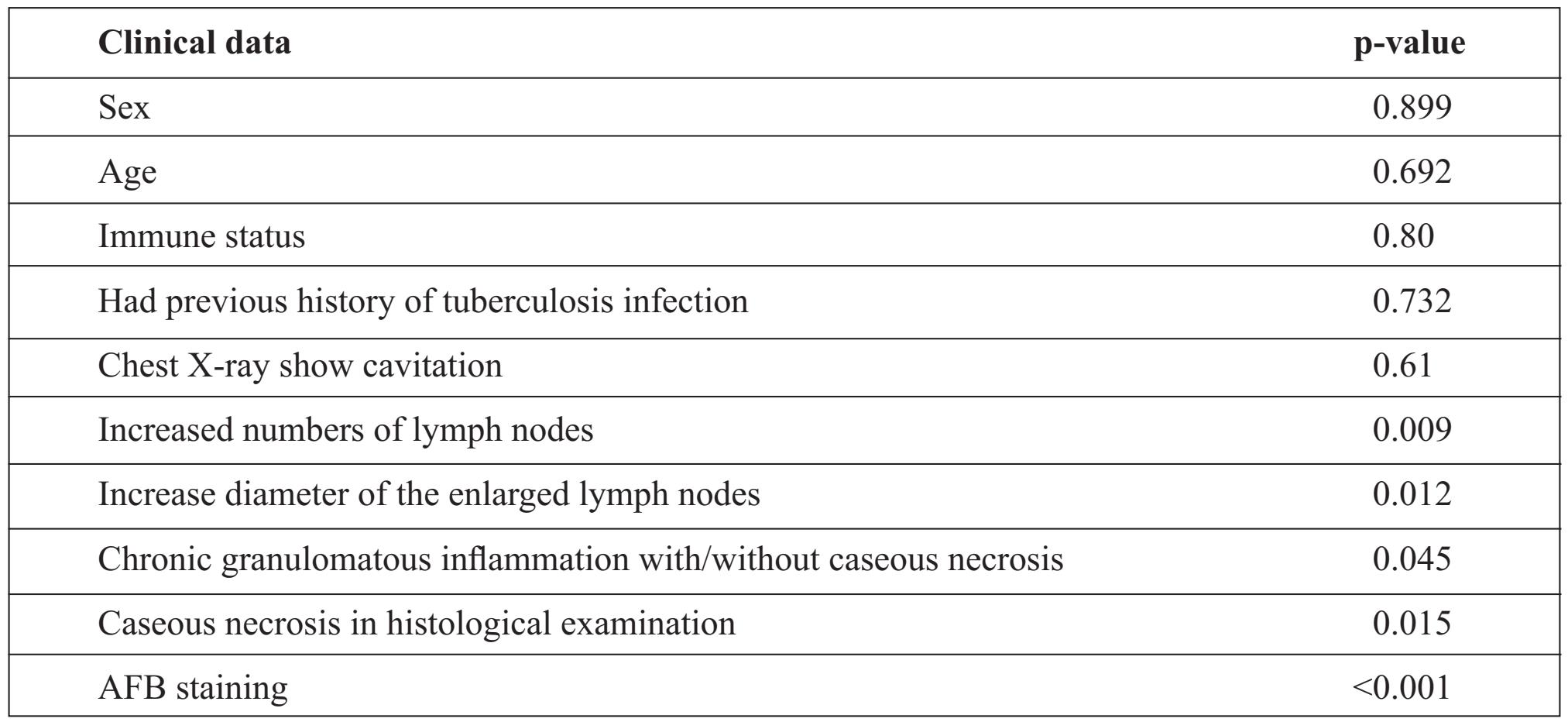
A LAMP+ had a significant correlation with: (a) the increased numbers of lymph nodes (p-value = 0.009), (b) the increased diameters of enlarged lymph nodes (p-value = 0.012), (c) chronic granulomatous inflammation with/without caseous necrosis (p-value = 0.045), (d) caseous necrosis seen in the histological examination (p value = 0.015), and (e) AFB staining (p-value <0.001) (Table 3).
Table 2 AFB staining and LAMP results in each lymph node study group.
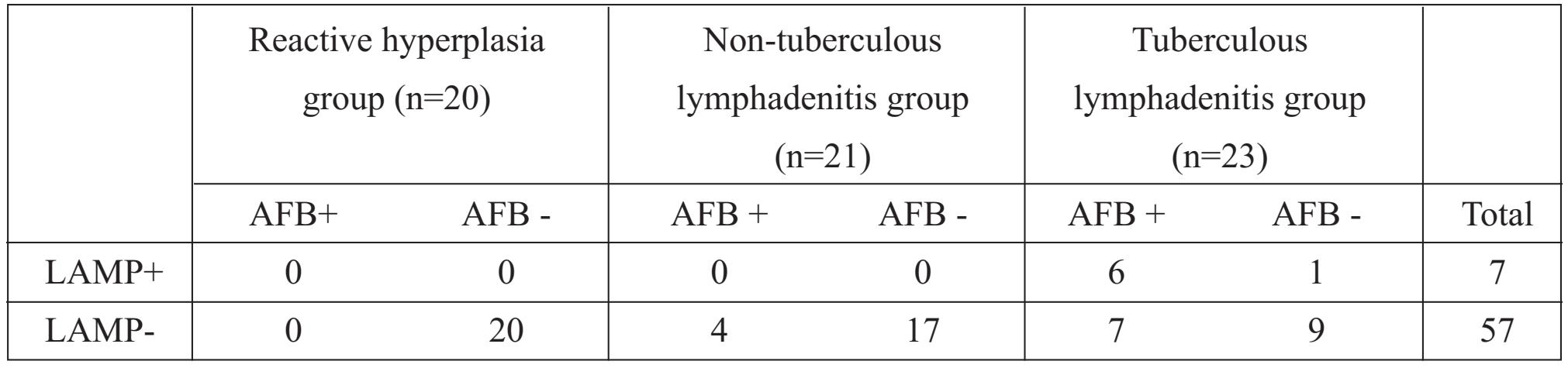
Table 3 LAMP results with clinical and laboratory data
DISCUSSION
In our study, LAMP had a positive correlation with the pathology of tuberculous lymphadenitis; viz., histological features, positive AFB staining, and the increased number and diameter of the nodes involved. These factors indirectly indicated that LAMP+ was related to an increased amount of M. tuberculosis in the sample. While DNA extraction from samples yielded a satisfactory amount of DNA, our LAMP assay had a high specificity but low sensitivity for detection of M. tuberculosis in FFPE lymph nodes. The LAMP assay usually has both a high sensitivity and specificity, because the amplification reaction occurs only when all six regions within a target DNA are correctly recognized by the primers. The low sensitivity of LAMP is possibly from bacterial DNA structural damage in some samples during tissue fixation and processing, and/ or there may have been Bst polymerase inhibitor in the tissue during DNA extraction.
Studies of LAMP detection of M. tuberculosis in fresh clinical specimens samples—sputum and CSF yielded respective sensitivities of 100% and 88.23% [8,11]. The high sensitivity in these studies is possibly due to the well-preserved M. tuberculosis DNA in fresh samples. As an alternative approach for the diagnosis of TB in FFPE, other studies applied PCR for the detection of M. tuberculosis in FFPE samples for a sensitivity of up to 66% [14] and 78%, and a specificity of 88% [15].
Our retrospective study suggests that AFB with a sensitivity of 56.5% is acceptable for routine practice since it is less expensive and common practice; however, for confirming a diagnosis or handling problematic cases in FFPE, PCR would be more appropriate than LAMP. The LAMP technique—an assay with high specificity, simple to do and able to provide a diagnosis in less than 2 hours—is, however, suitable for rapid diagnosis in outpatient care, for field work and in areas where culture is not available.
CONCLUSION
LAMP for diagnosis of TB in FFPE tissue had a high specificity, PPV and NPV but a low sensitivity. The results of the current study and previous studies indicate that LAMP is suitable for the study of fresh specimens.
Funding
This research was supported by Khon Kaen University Research Foundation, and The Invitation Research Fund from Faculty of Medicine, Khon Kaen University, Grant No. I54108.
Conflict of interest
None.
ACKNOWLEDGEMENTS
We thank (a) the staff at the Medical Records Section (b) the laboratory technicians for their meticulous work (c) Professor Shanop Shuang-shoti for detailed suggestions and (d) Mr. Bryan Roderick Hamman for assistance with the English-language presentation of the manuscript.
REFERENCES
1. Delogu G, Fadda G. The quest for a new vaccine against tuberculosis. J Infect Dev Countr 2009; 3(1): 5-15.
2. Lawn SD, Zumla AI. Tuberculosis. Lancet 2011; Jul 2; 378(9785): 57-72. Epub 2011 Mar 21.
3. Grange JM, Daborn C, Cosivi O. HIV-related tuberculosis due to Mycobacterium bovis. Eur Respir J 1994; 7(9): 1564-6.
4. Keshavjee S, Farmer PE. Tuberculosis, Drug Resistance, and the History of Modern Medicine. N Engl J Med 2012 Sep 6; 367(10): 931-6.
5. Perenboom Rm Fau-Richter C, Richter C Fau-Swai AB, Swai Ab Fau-Kitinya J, et al. Diagnosis of tuberculous lymphadenitis in an area of HIV infection and limited diagnostic facilities. Trop Geogr Med 1994; 46(5): 288-92.
6. Polesky A, Grove W, Bhatia G. Peripheral tuberculous lymphadenitis: epidemiology, diagnosis, treatment, and outcome. Medicine 2005; 84:350.
7. Tomita N, Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat protoc 2008; 3: 877.
8. Pandey BD, Poudel A, Yoda T, et al. Development of an in-house loop-mediated isothermal amplification (LAMP) assay for detection of Mycobacterium tuberculosis and evaluation in sputum samples of Nepalese patients. J Med Microbiol 2008; 57: 439-43.
9. Iwamoto T, Sonobe T, Hayashi K. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples. J Clin Microbiol 2003; 41: 2616-22.
10. Boehme CC, Nabeta P, Henostroza G, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol 2007; 45:1936-40.
11. Nagdev KJ, Kashyap RS, Parida MM, et al. Loop-mediated isothermal amplification for rapid and reliable diagnosis of tuberculous meningitis. J Clin Microbiol 2011 May; 49(5): 1861-5.
12. George G, Mony P, Kenneth J. Comparison of the efficacies of loop-mediated isothermal amplification, fluorescence smear microscopy and culture for the diagnosis of tuberculosis. PLoS One 2011; 6: e21007.
13. Arimatsu Y, Kaewkes S, Laha T, Hong SJ, Sri-pa B. Rapid detection of Opisthorchis viverrini copro-DNA using loop-mediated isothermal amplification (LAMP). Parasitol Int 2012; 61: 178-82.
14. Nopvichai C, Sanpavat A, Sawatdee R, et al. R detection of Mycobacterium tuberculosis in necrotising non-granulomatous lymphadenitis using formalin-fixed paraffin-embedded tissue: a study in Thai patients. J Clin Pathol 2009 Sep; 62(9): 812-5.
15. Park DY, Kim JY, Choi KU, et al. Comparison of polymerase chain reaction with histopathologic features for diagnosis of tuberculosis in formalin-fixed, paraffin-embedded histologic specimens. Arch pathol lab med 2003; 127: 32630.


