The role of Immunohistochemistry in diagnosis of renal cell carcinoma subtypes.
Sutthiporn Namnak, BSc *, Wipawee Kittikowit, MD *, Jutamas Wongphoom, BSc*
*Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Correspondence to: Wipawee Kittikowit, MD
Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
Email: fmedwkt@yahoo. com
ABSTRACT
Objectives: To determine the validity of immunohistochemistry in classifying Renal cell carcinoma (RCC) subtypes.
Methods: Eighty-three cases of RCC were retrieved. Immunohistochemical staining ofVimentin, CD10, CK7, P504s, Parvalbumin, TFE3, and CD 117 were done by using tissue microarray blocks. RCC subtypes were placed and compared with the previous diagnosis.
Results: Seventy-eight of 83 cases showed agreement with the original diagnosis. Vimentin expressions were observed in Clear cell RCC(CRCC) and Papillary RCC(PRCC). CD10 staining was detected in CRCC (82%) and in PRCC (77%) and Chromophobe RCC (CHRCC) (50%). CK7 was positive in all cases of CHRCC, and negative in 93% of CRCC. P504s was expressed in 88% of PRCC and negative in 96% of CRCC and 84% of CHRCC. Parvalbumin was expressed in 32% of CHRCC, and negative in all CRCC and PRCC. CD117 was positive in all CHRCC and negative in CRCC and PRCC. TFE3 was negative in all cases.
Conclusion: The expression patterns of the IHC markers in RCC subtypes were concluded. Vimentin, CD10 and CK7 were suggested as the first-stepped IHC markers. CD117, Parvalbumin, and P504s were suggested as the second stepped IHC markers to confirm the diagnosis.
Keywords: RCCs, Clear cell RCC, Papillary RCC, Chromophobe RCC, Xp11 translocation RCC, IHC
Introduction
Renal cell carcinoma(RCC) is the third most common cancer of the genitourinary tract and the most lethal urologic cancer, accounting for approximately 2% of adult malignancies and 90-95% of neoplasms arising from the kidney.[1] More than 90% of the renal cell carcinoma arise from the renal tubules[2]. Formally, RCC was divided into 5 main histologic subtypes: Clear cell, Papillary, Chromophobe, Collecting duct and unclassified RCC [3,23]. Clear cell, Papillary and Chromophobe RCC are the three most common types, comprising 70-80%, 14-17% and 4-8% of all RCCs, respectively. Collecting duct carcinoma is the rarest type of RCC(<1%). Unclassified RCCs include those that do not fit into any of the above 4 subtypes either morphologically or cytogenetically.[3,4]
Almost all malignant renal tumors arise from the renal tubules, collecting duct, or renal pelvic urothelium [5]. In the last few years, new types of RCC have been described, including Xp11 translocation renal cell carcinoma. Xp11 translocation RCC, a recently recognized distinct subtype is a rare tumor predominantly in young patients.
Adult renal epithelial tumors are heterogenous neoplasms. The World Health Organization (WHO) classification recognized a growing number of relevant clinicopathological subtypes with different pathogenetics and prognoses[6]. Although, in most cases, the diagnosis is based on morphological examination, cytogenetic changes, fusion transcript detection and immunohistochemical analysis are useful ancillary techniques to refine the classification [2,7]. In this study, we aimed to identify the IHC panel which would be reliable to increase precision of the diagnosis of the RCC subtypes.
MATERIAL AND METHODS
Tissue specimens:
All cases of renal cell carcinoma diagnosed between 2004 and 2009 were retrieved from the departments of pathology, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn memorial hospital. Eighty-three renal tumor cases obtained from patients treated by radical nephrectomy were included. All microscopic sections of these 83 cases were reviewed to select the proper tissue blocks for tissue microarray (TMA) by a pathologist. The previous diagnoses were recorded for comparison. The histological types were determined according to the WHO 2004 classification [6].
Immunohistochemical techniques:
Representative areas were identified on H&E slides and marked for sampling with TMAs, Using the manual TMA blocks, cores 4.0 mm in diameter were extracted from 1 to 2 paraffin-embedded tissue blocks from each case (one TMA block including four cases, 20 cores). All TMA tissue blocks were cut into 2 pm sections and placed on Superfrost Plus slides. The following antibodies were included in this study: CK7 (MU255-UC Clone OV-TL 12/30, BioGenex, CA, USA; dilution 1:100), CD10 (NCl-L-CD10-270 clone 56C6, Novocastra, LEICA,UK; dilution 1:25), P504s (Mob 438 Mouse anti P504s clone 13H4, Diagnostic BioSystem, CA, USA; dilution 1:800), Vimentin (M702U clone Vim 3B4, DAKO, Denmark; dilution 1:200), Parvalbu-min (AB50338, Mouse monoclonal clone PARV-19, Abcam, UK; dilution 1:2000), TFE3 (TFE3(P-16) sc-5958, goat polyclonal IgG, SantaCruz Biotechnology, CA, USA; dilution 1:4000) and CD117(rabbit polyclonal, DAKO, Denmark; dilution 1:500). Sections were then processed in a Ventana automated immunostainer (Ventana, Tucson, AZ) for 6 antibodies and using Ultraview Universal DAB detection Kit (Ventana, Tucson, AZ) according to the manufacturer’s instructions for visualization. For TFE3 antibody, manual immunohistochemical staining was applied.
Tumor cells were considered positive only when the appropriate staining patterns were achieved (CD10 gives cell surface staining, CK7 gives cytoplasmic and membranous staining, Vimentin gives cytoplasmic staining, P504s gives cytoplasmic staining, Parvalbumin gives cytoplasmic staining, TFE3 gives nuclear staining and CD117 gives cell membrane staining). The extent of immunoreactivity was categorized into levels as (0), no positive tumor cell; (1+), positive cell <10%; (2+), positive cell 10-50% and (3+), positive cell >50%; and defined score 0 and 1+ as negative and score 2+ and 3+ as positive [8]. With combination of the IHC markers together with morphology, diagnoses were redone case by case. Then the new diagnoses were compared with the previous ones.
RESULT
Seventy-eight from 83 cases, the diagnoses of the RCC subtypes were concordant, which consisted of 55 CRCC, 17 PRCC and 6 CHRCC. Disagreement in diagnosis was found in 5 cases, some of which the diagnoses were not achieved with IHC. Thereafter, we used CD117 antibody as an additional immunostaining for supporting the result.
The results of immunohistochemical staining in 78 cases were detailed (Table 1). Representative H&E and IHC staining was illustrated (Figure 1).
Table 1 The results of Immunohistochemical staining
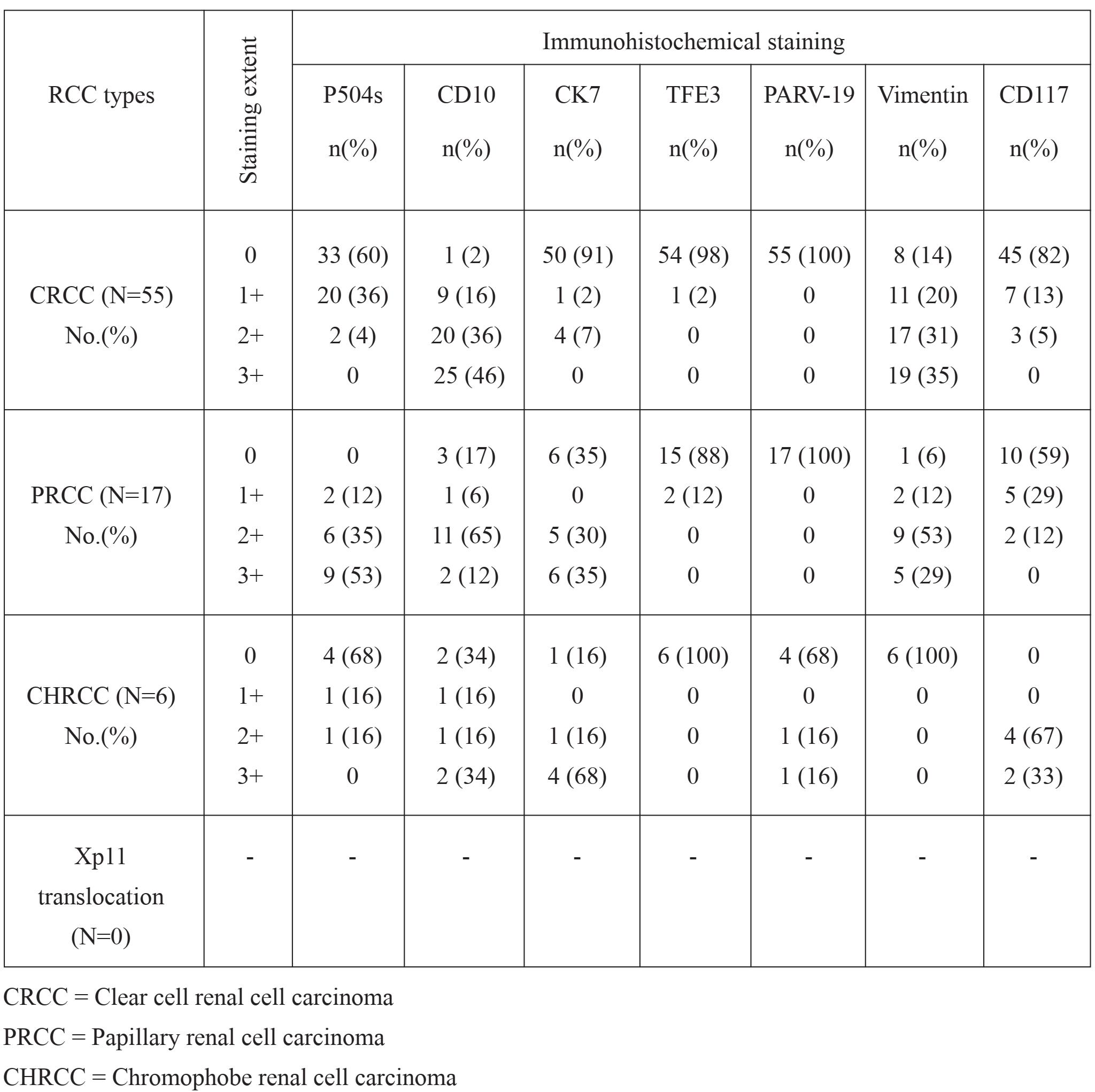
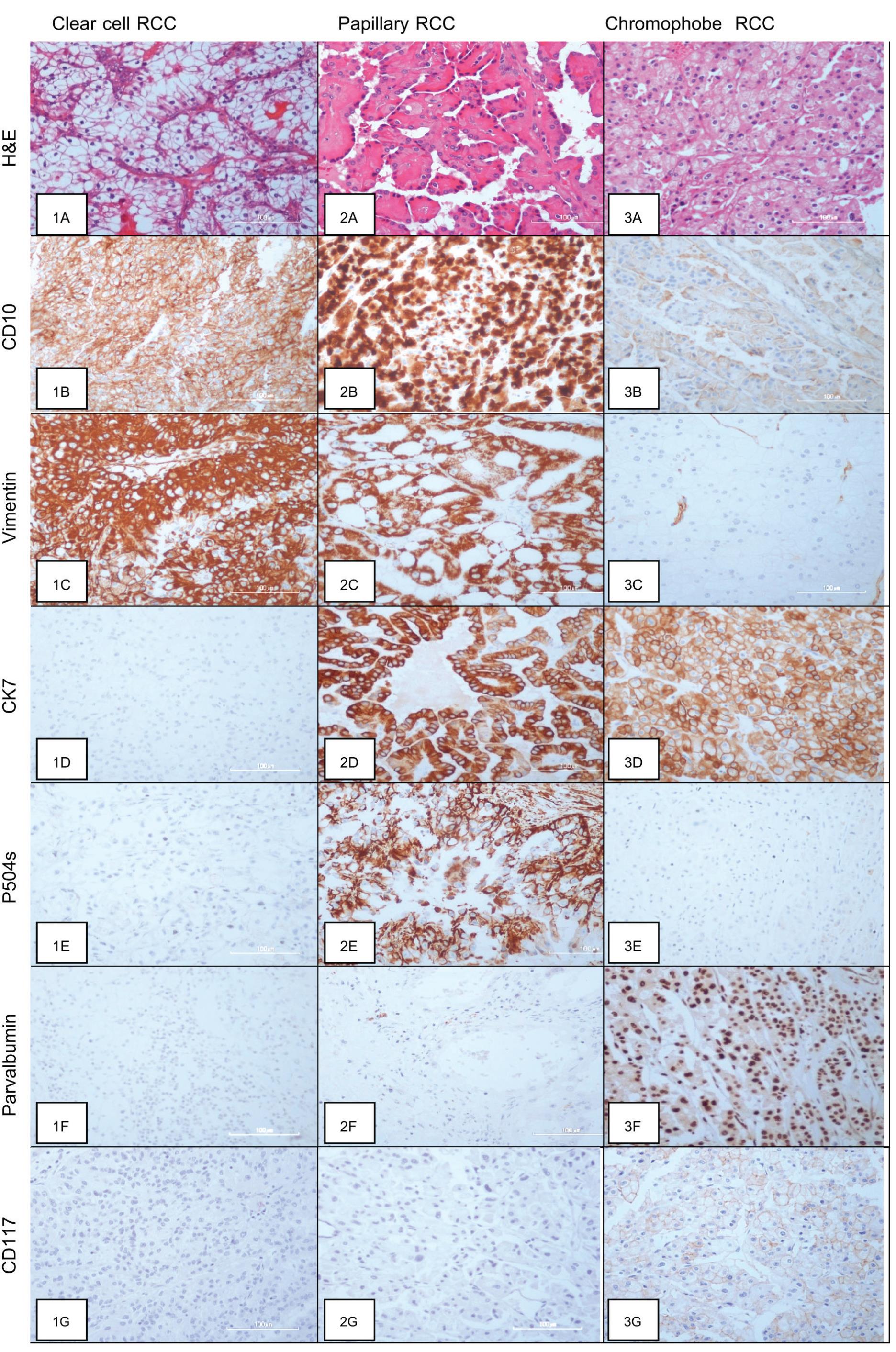
Figure 1 The microscopic feature and Immunohistochemical illustrations
Histomorphology of Clear cell RCC (1A), Papillary RCC (2A) and Chromophobe RCC (3A); Strongly positive staining of CD10 along the cytoplasmic border in CRCC (1B), in the cytoplasm of PRCC (2B) and weakly staining in some cases of CHRCC (3B); Strongly positive staining of Vimentin in the cytoplasm of CRCC (1C) and PRCC (2C) but negative in CHRCC (3C); Negative staining of CK7 in CRCC (1D), positive staining of CK7 in the cytoplasm of PRCC (2D) and CHRCC (3D); P504s positive in the cytoplasm of PRCC (2E) and negative in CRCC (1E) and CHRCC (3E); Negative staining of Parvalbumin in CRCC (1F) and PRCC (2F) and positive in CHRCC with more intensity in the nucleus than the cytoplasm (3F); Negative staining of CD117 in CRCC (1G) and PRCC (2G) but positive staining in the cytoplasmic membrane of CHRCC (3G)
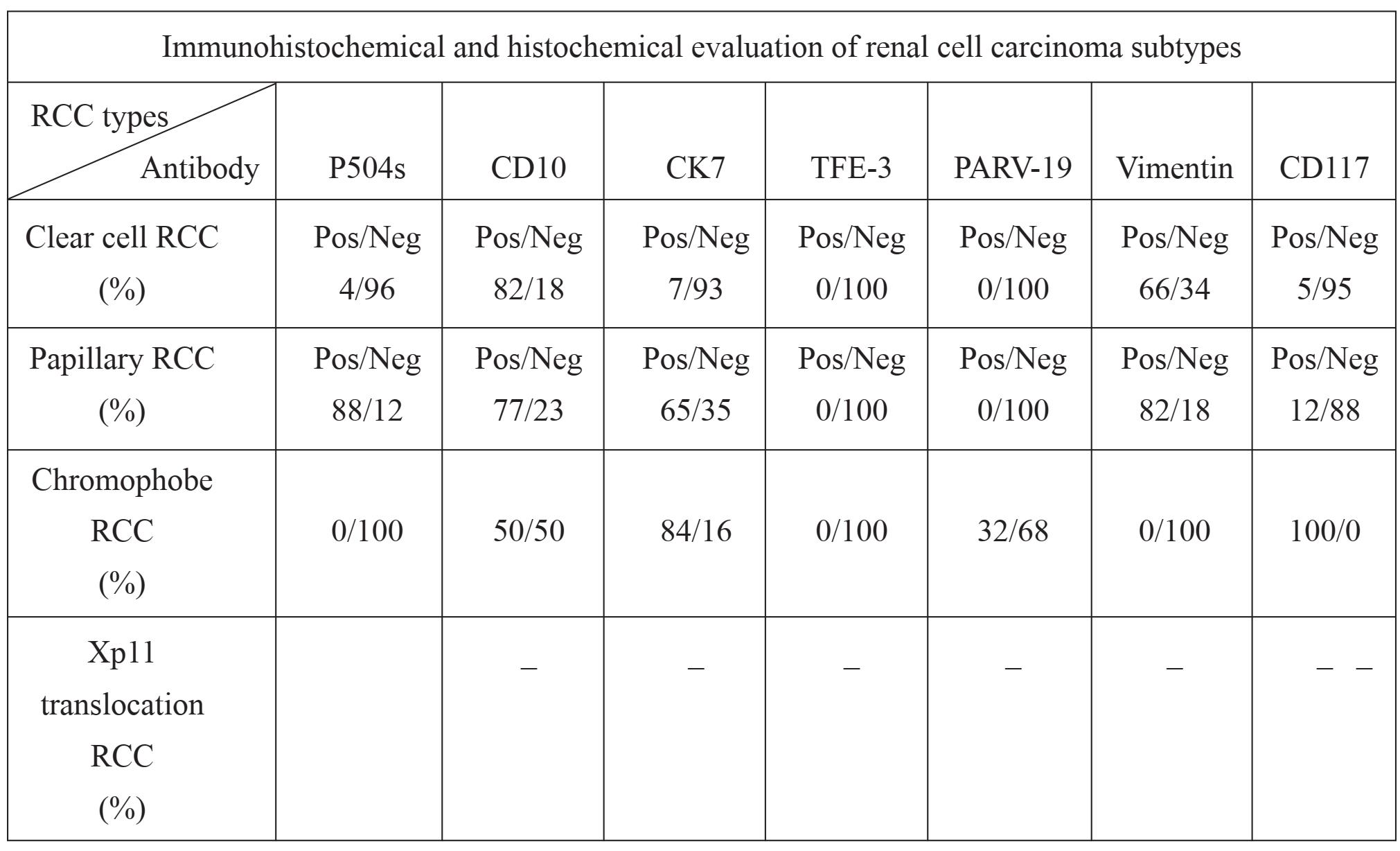
CD10 staining was expressed in most (45/55; 82%) CRCC. CD10 expression was also observed in 77% (13/17) of PRCC and 50% (3/6) of CHRCC. Vimentin staining was absent in all CHRCC, whereas diffuse cytoplasmic staining was presented in 36 of 55 CRCC (66%) and in 14 of 17 PRCC (82%). Five cases (84%) of CHRCC showed cytoplasmic staining with membrane accentuation for CK7, while CRCC were negative in 93% (51/55). CK7 expression was also observed in 11 of 17 cases (65%) of PRCC. None of the CRCC and PRCC showed Parvalbumin staining. Positive cytoplasmic and nuclear staining was present in 2 of 6 (32%) CHRCCs. Immunoreactivity for P504s was presented in 15 of 17 (88%) PRCC. P504s staining was absent in 5 of 6 (84) CHRCC and faintly expressed in 4% of CRCC (2/55). TFE3 was nonreactive in all cases. In addition, CD117 was positive in all CHRCC with moderate intensity. The staining was complete membranous pattern. In contrast, all CRCC and PRCC were negative for CD117. (Table 2)
Table 2 The summary of Immunohistochemical staining
DISCUSSION
The usefulness of IHC staining for discriminating subtypes of RCC was documented [2,7,9,10 ]. We use 6 antibodies as the first-stepped immu-nopanel. The expression patterns of the IHC panel in most cases of each subtype were summarized as follow: CRCC-- Vimentin (+), CD10 (+), and CK7 (-); PRCC--Vimentin (+), CD10 (+), CK7 (+), and P504s (+); and CHRCC-- Vimentin (-), CD10 (±), CK7 (+), Parvalbumin (+). Our results were agreed with the studies of Bazille et al [9] and Tretiakava et al [11] who found that Vimentin was positive in CRCC and negative in CHRCC; and P504s was presented in 88% of PRCC, 4% of CRCC, and absent in CHRCC, respectively. These results were agreed with the studies of Lin et al [12] and Allory et al [13]. It also supported the study of Zhou et al [14] of which expression of CD10 was found in 94-100% of CRCC and in 67-93% of PRCC. Invariable expressions .....in CHRCC and negative staining in CRCC
of CK7 were documented in the studies by Adley et al [15]and Skinnider et al [16]. Parvalbumin was expressed in only CHRCC as many as 80% of cases in previous studies [17,18]. The diagnoses were unable in some of the five cases with incongruent diagnoses. The immunoprofiles of these cases were not applicable to any subtype. While some cases, the immunoprofiles were disagreed with the morphology. Additional immunostaining, CD117 were applied in the second step to support the diagnosis of CHRCC [7]. The study by Pan et al [19] and Wang et al [20] discovered the expression of CD117 in CHRCC, while the other subtypes were non-reactive . None of our cases were positive with TFE3 markers. The TFE3 was specific for Xp11 translocation RCC, which was recently added as a new subtype of RCCs [6]. Therefore, incidence of the Xp11 translocation RCC could not be determined in this study. However, other subtypes oftranslocation RCC, such as t(6;11)(p21;q13) translocation were not completely excluded. This type of tumor could be stated by detection of TFEB expression [21].
We recommended using Vimentin, CK7, and CD10 as the first-stepped IHC panel. Then the second step, IHC would be used to confirm subtypes. We suggested P504s (to confirm PRCC), CD117 and Parvalbumin (for CHRCC). However, there were cases which the morphologic features and the immunoprofiles were incongruent. According to the recent WHO classification of the renal cell carcinoma subtypes, genetic derangement of the tumor was the most reliable information to determine the RCC subtype [6]. Therefore, cytogenetic means should be the most appropriate or gold standard to use in diagnosis [22]. However, the cytogenetic methods were costly and inapplicable in our study or even routine pathological laboratory service. Immunohistochemical studies are still an appropriate and advantageous techniques in providing information for accurately categorizing most cases of the renal cell carcinoma.
REFERENCES
1 Motzer RJ, Bander NH, Nanus DM, Renal-cell carcinoma. N Engl J Med 1996; 335: 865-875.
2 Truong LD, Shen SS. Immunohistochemical Diagnosis of Renal Neoplasms. Arch Pathol Lab Med 2001; 135: 92-109.
3 Ljungberg B, Alamdari FI, Stenling R, Roos G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur Urol 1999; 36: 565-569.
4 Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Patho 2003; 27: 612-624.
5 Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000; 164: 1523-1525.
6 Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. France: IARC press 2004; 37-38.
7 Liu L, Qian J, Singh H, Meiers I, Zhou X, Bostwick DG. Immunohistochemical analysis of Chromophobe renal cell carcinoma, Renal oncocytoma, and Clear cell carcinoma. Arch Pathol Lab Med 2007; 131: 1290-1297.
8 Zhang Y, Li Q, Zhu F. Subcellular localization of APMCF I and its biological significance of expression pattern in normal and malignant human tissue. J Exp Clin Cancer Res 2009; 21: 111-116.
9 Bazille C, Allory Y, Molinie V, et al. Immu-nohistochemical characterization of the main histologic subtypes of epithelial renal tumours on tissue-microarrays:study of 310 cases[in French]. Ann Pathol 2004; 24: 395-406.
10 Molinie V, Balaton A, Rotman S, et al. Alpha-methyl CoA racemase expression in renal cell carcinomas. Human Pathology 2006; 37: 698703.
11 Tretiakova MS, Sahoo S, Takahashi M, et al. Expression of alpha-methylacyl-CoA racemase in papillary renal cell carcinoma. Am J Surg Pathol 2004; 28: 69-76.
12 Lin F, Brown RE, Shen T, Yang XJ, Schuerch C. Immunohistochemical detection of P504s in primary and metastatic renal cell carcinomas. Appl Immunohistochem Mol Morphol 2004; 12:153-159.
13 Allory Y, Bazille C, Vieillefond A, et al. Profiling and classification tree applied to renal epithelial tumours. Histopathology 2008; 52: 158-166.
14 Zhou M, Roma A, Galluzzi CM. The usefulness of immunohistochemical markers in the differential diagnosis of renal neoplasms. Clin Lab Med 2005; 25: 247-257.
15 Adley BP, Papavero V, Sugimura J, The BT, Yang XJ. Diagnostic value of cytokeratin 7 and parvalbumin in differentiating chromophobe renal cell carcinoma from renal oncocytoma. Anal Quant Cytol Histol 2006; 28: 228-36.
16 Skinnider BF, Folpe AL, Hennigar RA, et al. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: Potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol 2005; 29 : 747-754.
17 Martignoni G, Pea M, Chilosi M, et al. Parval-bumin is constantly expressed in chromophobe renal carcinoma. Mod Pathol 2001; 14: 760767.
18 Abrahams NA, MacLennan GT, Khoury JD, et al. Chromophobe renal cell carcinoma: a comparative study of histopathological, immuno-histochemical and ultrastructural features using high throughput tissue microarray. Histopathology 2004; 45:593-602.
19 Pan CC, Chen PC, Chiang H. Overexpression of KIT(CD117) in chromophobe renal cell carcinoma and renal oncocytoma. Am J Clin Pathol 2004; 121: 878-883.
20 Wang HY, Mills SE. KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. Am J Surg Pathol 2005; 29:640-646.
21 Argani P, Ladanyi M. Translocation Carcinoma of the Kidney. Clin Lab Med 2005; 25: 363-378.
22 Roh MH, Cin PD, Silverman SG, Cibas ES. The Application of Cytogenetics and Fluorescence In Situ Hybridization to Fine-Needle Aspira-
tion in the Diagnosis and Subclassification of Renal Neoplasms. Cancer cytopathology 2010; 25: 137-145.
23 Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumors. Journal of Pathology 1997;183:131-133.


