Increased eosinophil levels in HIV-infected patients with high CD4/CD8 ratio Study from 564 patients in Ramathibodi Hospital.
Preyarat kaewketthong*, Jaruwan Doydee*, Supachewin Thawenuch*, Thanakorn Thuayset*,
Kalayanee Khupulsup** and Punnee Butthep*
*Hematology Division, **Immunology Division, Department of Pathology, Faculty of Medicine Ramathibodi
Hospital, Mahidol University, Bangkok 10400, Thailand
Correspondence address: Punnee Butthep, PhD.
Department of Pathology, Faculty of Medicine Ramathibodi Hospital
Rama 6 Road, Bangkok, 10400 Thailand
Tel./Fax. 662- 2011378 E-mail: punnee.but@mahidol.ac.th
ABSTRACT
Blood from 564 patients infected with human immunodeficiency virus (HIV) were studied for laboratory analysis. The hematological changes including white blood cell count (WBC), percentage and absolute number of lymphocyte and eosinophil were determined by using the Technicon H*3 RTX hematological analyzer. Analysis of blood cell parameters were done within 4 hours after venous blood collection. It was noted that 201(35.6%) out of 564 patients had eosinophilia and 148 (73.6%) out of those 201 patients with eosinophilia showed increased CD4/CD8 ratio as compared with those in patients with normal eosinophil. Furthermore, we found that the patients with extremely high eosinophilia (greater than 60% eosinophil) had the greatest degree of increased CD4/CD8 ratio. Significant correlation between % eosinophil and absolute CD4 (r = 0.552, p-value = < 0.0001) and between % eosinophil and CD4/CD8 ratio (r = 0.716, p-value = < 0.0001) were also observed. It may be suggested that eosinophil estimation might be used as a simple marker from blood smear observation for an assessment of patient status which could be related to the CD values and possibly prognosis of the HIV-infected patients.
Keywords: Eosinophil, Eosinophilia, HIV, CD4, CD8, CD4/CD8 ratio
Short running title: Eosinophilia in HIV-infected patients
INTRODUCTION
A unique human retrovirus termed human immune deficiency virus (HIV) is the primary etio-logic agent in the pathogenesis of the acquired immune deficiency syndrome (AIDS) [1-2]. Many categories of clinical outcome are recognized after infection with HIV: generalized unexplained lym-phadenopathy, persistent fever, weight loss, unexplained diarrhea, central nervous system dysfunction, and asymptomatic carrier detected by specific serum antibodies to HIV. Several studies have described the hematologic effects including both in peripheral blood and bone marrow of the HIV-infected patients. Decreased blood cell counts are common manifestation of HIV infection. In addition to the characteristic decrease in CD4- positive T lymphocytes, anemia occurs in approximately 70%, granulocytopenia in 50%, thrombocytopenia in 40% and lymphopenia in 70% of HIV-infected patients [3-8]. These blood cell changes are caused by the direct effect of HIV infection or by the coexistent infections and the myelosuppressive drugs used in the treatment. Over the last decade, most of the researches have focused on the unrelenting force of HIV to attack the CD4 cell receptor, to enter the CD4 cell cytoplasm, to use HIV viral reverse transcriptase to insinuate itself into human DNA, and to eventually destroy CD4 cells. However, another cell line, especially eosinophil cell line, may also be abnormal in HIV-infected patients and an increase in eosinophil number and activation of eosinophil may also be important to the patients [9-10]. Eosinophils are produced in bone marrow from pluripotential stem cells. Three cytokines including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are particularly important in regulating the development of eosinophils [11]. Eosinophilia always occurs as a result of many processes such as differentiation of progenitor cells and proliferation of eosinophils in the bone marrow. However, eosinophilia can occur in a variety of disorders and can be classified as mild (351-1500 cells/cumm), moderate (>1500-5000 cells/cumm), or severe (>5000 cells/cumm) [11]. Eosinophilia may be classified as primary or secondary phenomenons. In primary eosinophilia, the increased production of eosinophils is due to an abnormality in a hematopoietic stem cell, for example, in eosinophilic leukemia. In secondary eosinophilia, the increased production of eosinophils is a reactive process driven by cytokines such as that occurs in the case of allergy. Increased eosinophil levels were also reported in HIV infected patients with and without specific cutaneous diagnosis and parasitic infection [12-13]. In addition, HIV infection itself may induce proliferation of eosinophils while other cell components are declining [14]. The objective of this study is to assess the eosinophil levels in HIV infected patients and to compare the eosinophil levels with white blood cells (WBC), % and absolute lymphocytes including absolute CD4, absolute CD8 and CD4/ CD8 ratio and to use as a marker of immune activation among the HIV infected patients.
PATIENTS AND METHODS
Patient population
Blood samples from 564 patients with HIVpositive were collected from the Department of Pathology Faculty of Medicine Ramathibodi Hospital, Mahidol University. The samples were studied for laboratory investigation including anti-HIV, lymphocyte subsets and hematological analysis. The patient population included 5 boys, 20 female adults and 539 male adults. The age of boy subjects was between 2-5 years old, whereas the average age of male and female adults was 25 years old (range 17-42 years old). Ethical approval was obtained from the Committee on Human Rights Related to
Researches involving Human Subjects of Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
HIV testing and determination of lymphocyte subsets
Sera from all patients were tested for antibodies against HIV using ELISA technique and gelatin particle agglutination test. The criteria for diagnosis followed the guidelines for HIV testing strategies for diagnosis of HIV infection [15]. The determination of percentages and absolute counts of human total T helper in erythrocyte-lysed whole blood using BD TriTEST™ three-color direct immunofluorescence reagents (BD Biosciences, San Jose, CA USA) on the FACSort (Becton Dickinson Biosciences FACSort).
Hematological analysis
The hematological parameters were analyzed by using Siemens Technicon H*3 automated hematology analyzer (Bayer Diagnostics USA) within 4 hours after venous blood collection using EDTA as an anticoagulant. The Technicon H*3 blood analyzer combined the capabilities of routine CBC and five-part differential blood analyzer with absolute lymphocyte count (Abs. lymph), and absolute eosinophil count (Abs. Eos). The Technicon H*3 included flow cytometric analysis of cells with laser light scattering to quantify cell volume, hemoglobin concentration, and light absorbance of cells stained with Oxazine 750 was employed to detect reticulocyte and distinguish them from mature red blood cell [16-17].
Statistical analysis
Results were analyzed and expressed as the mean ± standard deviation (Mean ± SD) and were compared among various eosinophil levels. Statistical differences between groups were tested with a non-parametric method (Wilcoxon sighed rank test, ANOVA). Spearman correlation analysis was done to determine the correlation coefficients among the quantitative data. Differences were considered significant when the p values were < 0.05.
RESULTS
The studies include data from 564 HIV-infected patients for hematological analysis, CD4, CD8 and CD4/CD8 ratio as compared with the eosinophil levels in the peripheral blood. About 95 percent of the patients were 17 to 42 years of age. Table 1 showed the numbers and the % of total of HIV infected patients enrolled in the studied as compared with eosinophil levels. We found that 64.4% of HIV-infected patients had normal level of peripheral blood eosinophil, whereas the other 35.6% had high level of eosinophil of which 28.8% had eosinophil in the range of 8-20%, 6.1% had 21-40% and 0.7% had 41-65%. Table 2 showed the hematologic parameters including white blood cells (WBC), % lymphocyte (Lymph), absolute lymphocytes (Abs. Lymph), % eosinophil (Eos) and Absolute eosinophil (Abs. Eos) as compared with the levels of eosinophil distribution. The WBC in peripheral blood of high eosinophil patients did not show any significant difference as compared with the other group, whereas low level of WBC was observed in the group of 41-50 % eosinophil patients. However, the highest level of WBC was demonstrated in the highest level of eosinophil group. In addition, the percentage of lymphocyte and Abs. lymph did not show any significant difference among the all groups. Table 3 showed the incidence of eosinophil distribution as compared with the levels of Abs. CD4, Abs. CD8 and CD4/CD8 ratio. The highest level of CD4/CD8 ratio (0.40 ± 0.02) was demonstrated in the patients with highest level of eosinophil (51- 65%), whereas the lowest CD4/CD8 ratio (0.05 ± 0.003) was demonstrated in the low to normal level of eosinophil.
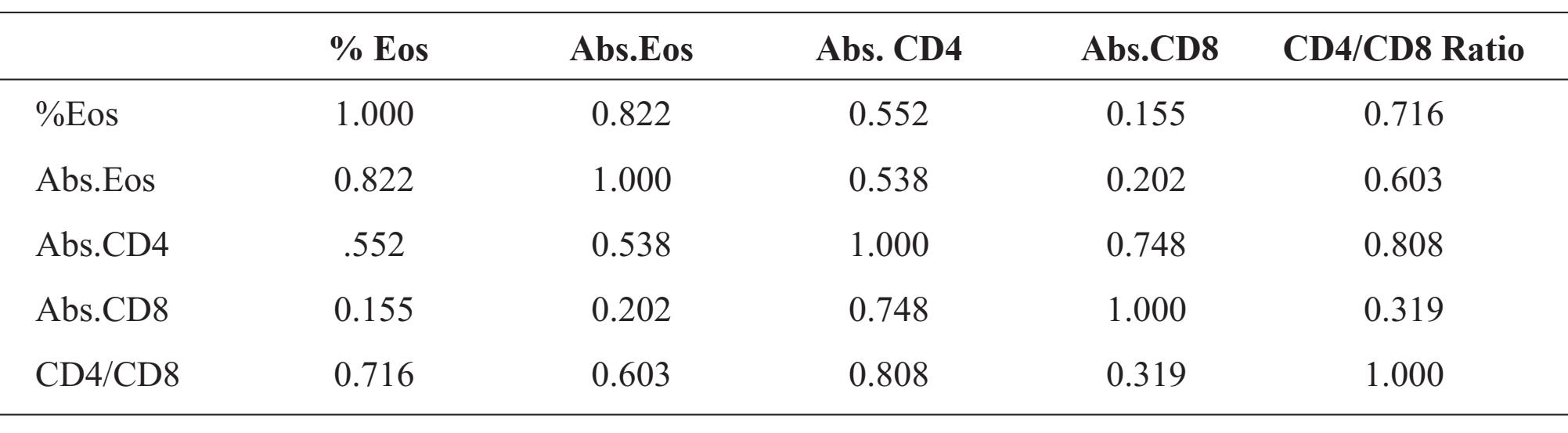
The correlation coefficients (r) among the percentages of eosinophil and Abs. Eos, Abs. CD4, Abs. CD8 and CD4/CD8 ratio were shown in Table 4. The statistical analysis of correlation coefficients (r) was demonstrated in figure 1A - 1D : r = 0.822, p-value = <0.0001 for % Eos vs Abs. Eos; r = 0.552, p-value =<0.0001 for % Eos vs Abs. CD4; r = 0.155, p-value = 0.0002 for % Eos vs Abs. CD8 and r = 0.716, p-value =< 0.0001 for % Eos vs CD4/CD8 ratio, respectively.
Table 1 Comparison between Eosinophil levels, number of HIV infected patients and percent of total patients enrolled in the study.
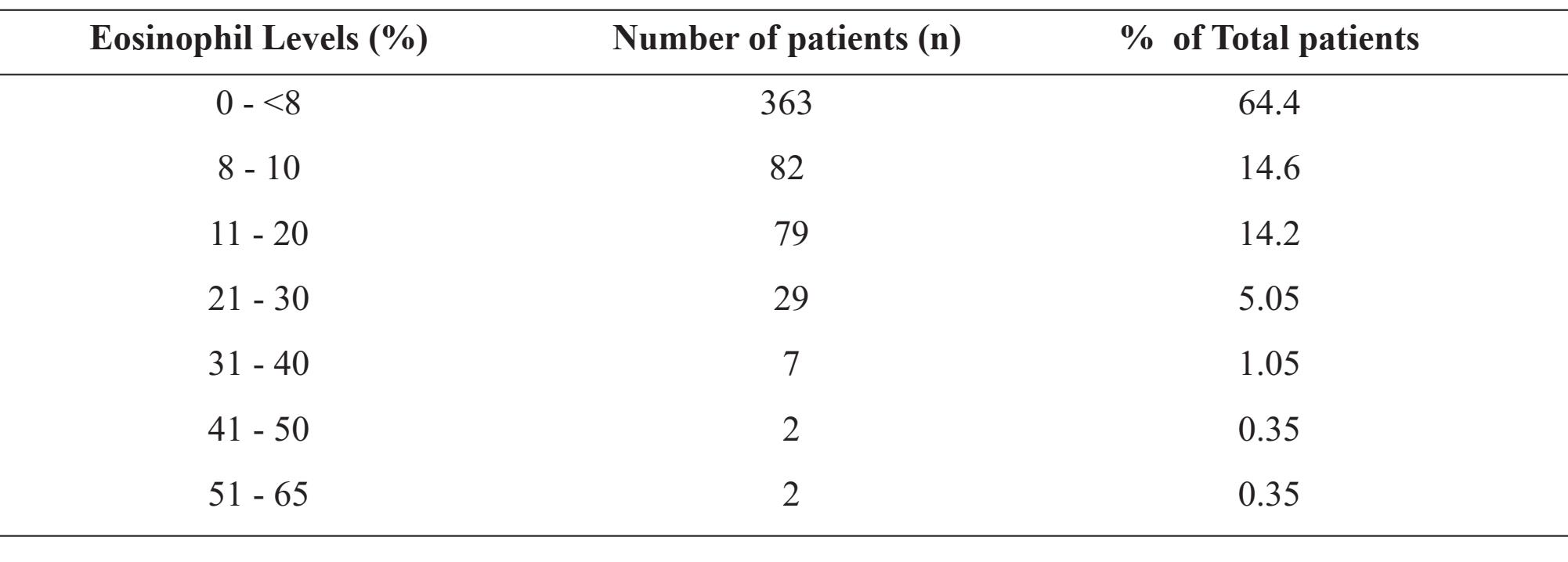
Table 2 Mean ± SD of WBC count, % and Abs. Lymphocyte (Abs. Lymph), % and Abs. Eosinophil (Abs. Eos) as compared among various levels of eosinophil distribution in peripheral blood of HIV infected patients.
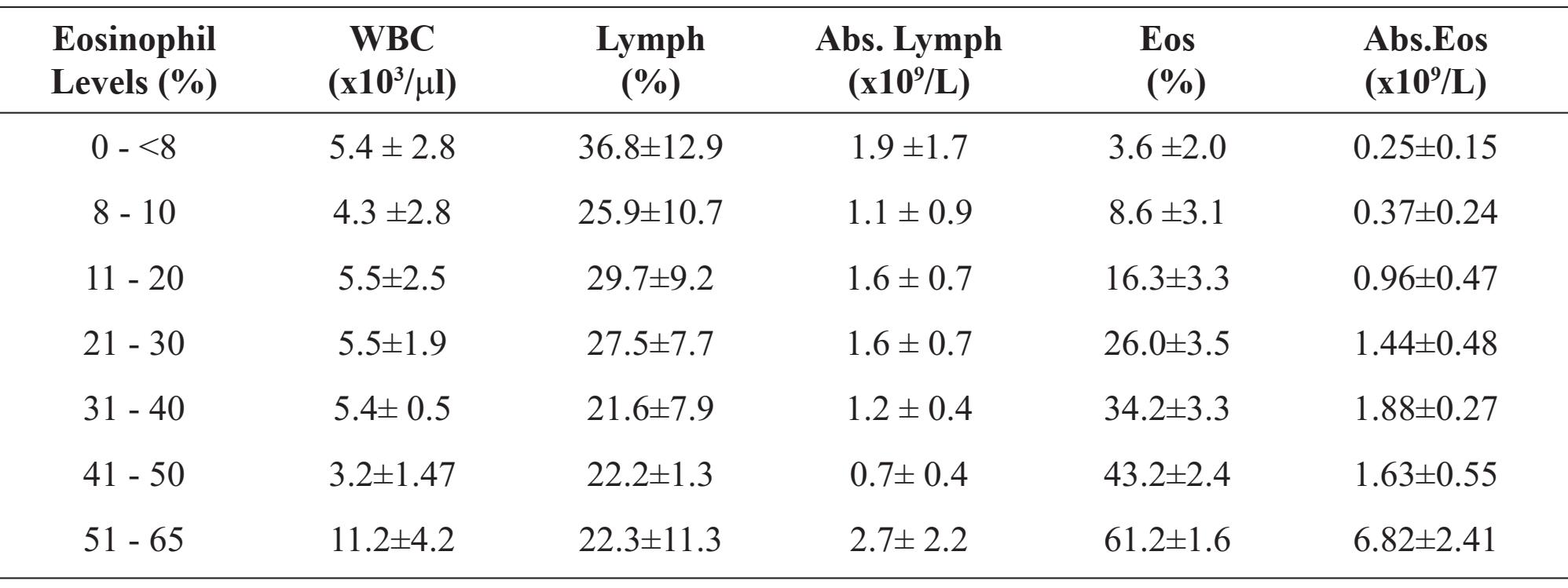
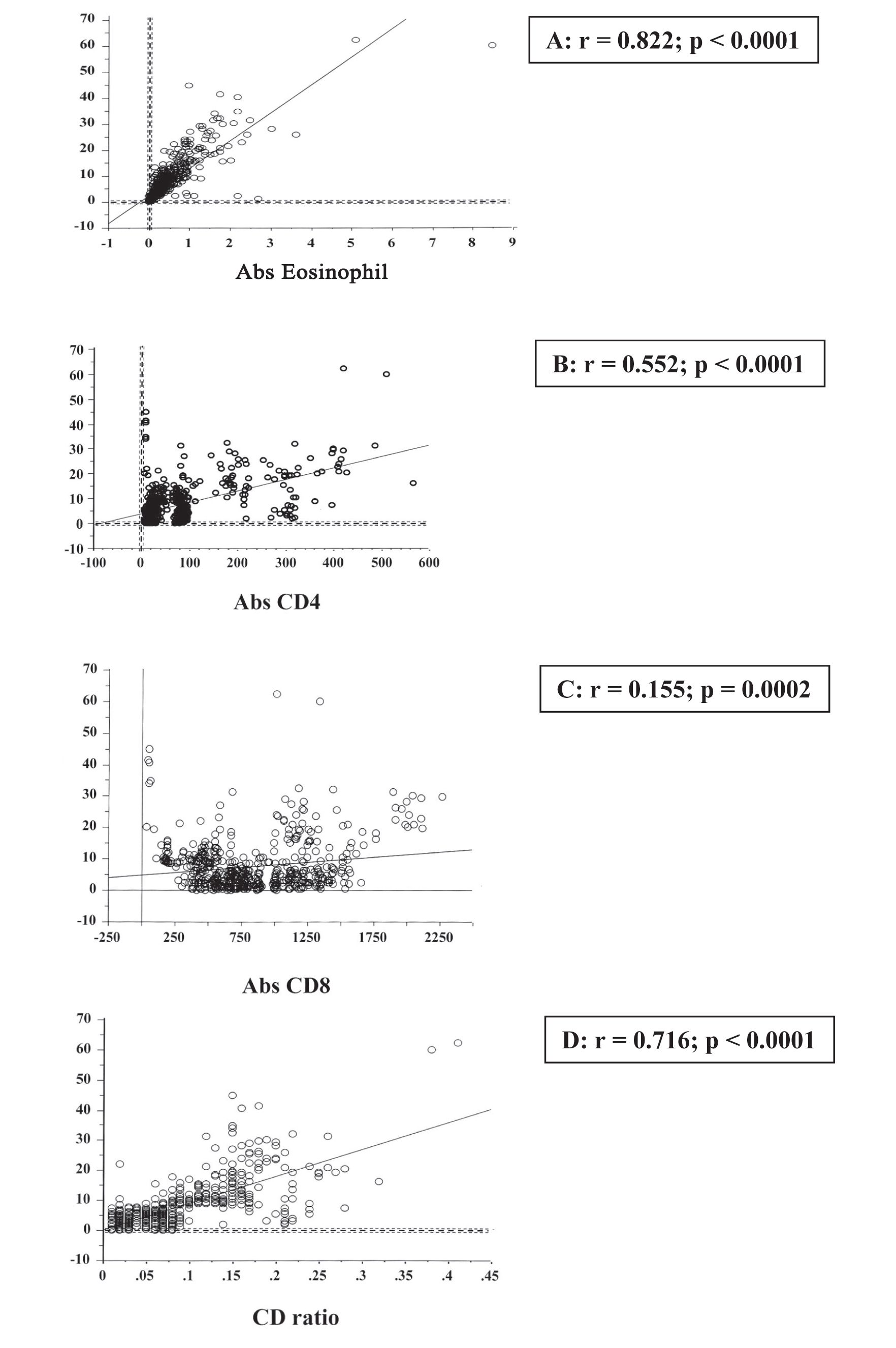
Figure 1 Correlation coefficient (r) between % Eosinophil versus Abs.Eos (A), Abs.CD4 (B), Abs.CD8 (C) and CD4/CD8 ratio (D).
Table 3 Mean ± SD of Abs.CD4, Abs.CD8 and CD4/CD8 ratio as compared among various levels of eosinophil distribution in peripheral blood of HIV infected patients.
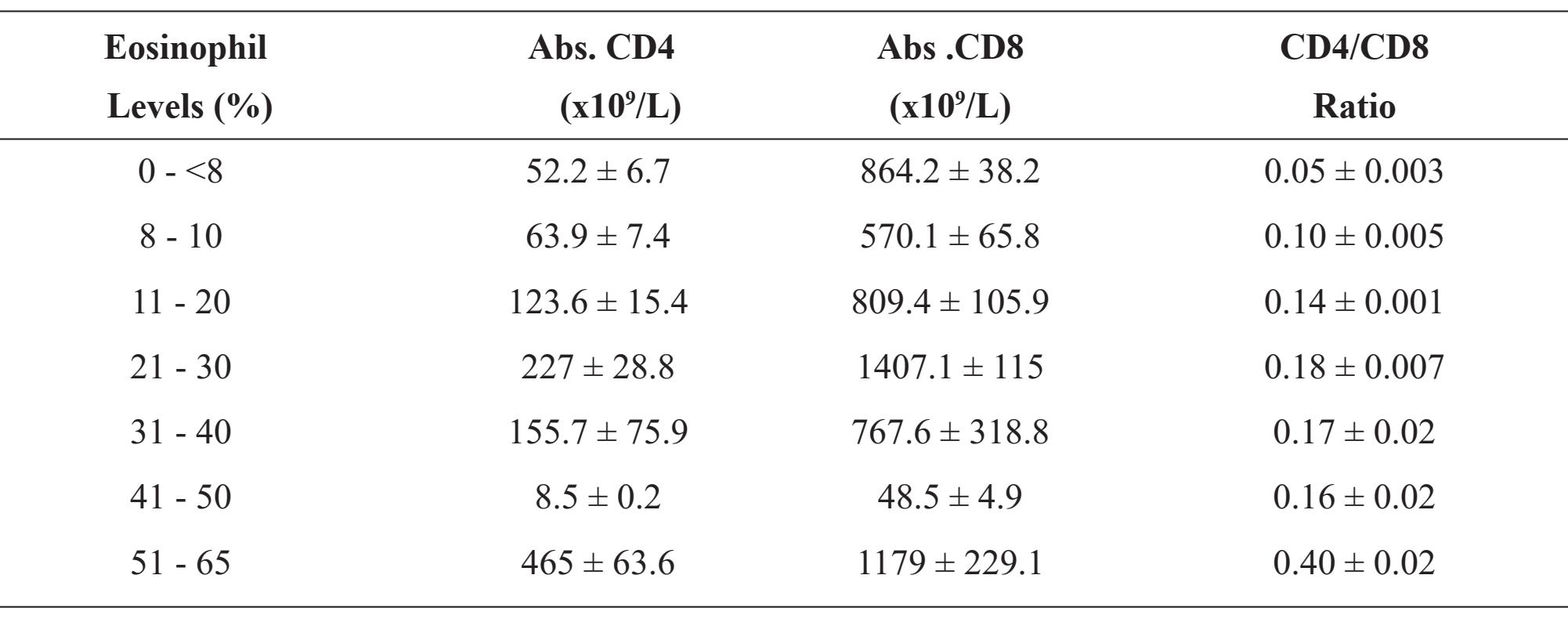
Table 4 Correlation coefficient (r) among % Eosinophil (% Eos), Abs.Eos, Abs.CD4, Abs.CD8 and CD4/CD8 ratio
DISCUSSION
There have been previously reported that eosinophilia is associated with HIV infection [1819]. Eosinophilia is defined as an eosinophil count > 0.4 x 109 cells/L [20]. In this study, we have shown that CD4/CD8 ratio was significantly higher in the HIV-infected patients with eosinophilia than those without eosinophilia. Although the ratio was lower than normal since the % differential of normal eosinophil was zero to less than 8% and normal value of CD4/CD8 ratio was 0.9- 3.6.
The CD4 is designed as T helper, while the CD8 is designed as T suppressor/ cytotoxic lymphocytes. According to the numbers of cells developing in the bone marrow, the physiological activity of eosinophils including cell surface receptor expression, degranulation and reactive oxygen radical production can be enhanced by the effects of granulocyte - macrophage colony-stimulating factor (GM-CSF), interleukin 5 (IL-5), and interleukin 3 (IL-3) [21-23]. These effects are probably responsible for the altered physiology of eosinophils in eosinophilia. Besides, IL-5 which plays an important role in selective eosinophilia, CD4+T cells in the various events also activate eosinophil recruitment. Recently, Kaminsky et al. [24] reported that 70.8% of HIV - infected patients with high CD4 cells have eosinophilia with or without parasitic infection. Nevertheless, the average percentages of eosinophils with parasitic infection are only 22% for hookworm, 11% for trichuris, 12% for ascaris and 13.2% for strongyloides, respectively.
Even though there were many studies showing correlation of eosinophilia in HIV infected patients with low CD4 count and some with late-stage [9,12-13,25-26]. Another study showed that eosinophil was associated with risk of progression to active TB, male gender, low socio-economic status, high CD4+ T-cell counts, and schistosomiasis [20]. The data from our study showed significant correlation between eosinophilia and Abs CD4 (r = 0.552; p < 0.0001) and also the significant correlation with the CD4/CD8 ratio (r = 0.716; p < 0.0001). We postulated that the HIV-infected patients with eosinophilia who have increased CD4/ CD8 ratio may have a better prognosis as compared with those patients who have low CD4/ CD8 ratio. However, the correlation with clinical outcome from our study was not possible due to unavailable clinical data.
The correlation between percentage of Eosinophil and CD4 lymphocytes and CD4/ CD8 ratio might refer to the immunological response in these patients, so the eosinophil estimation from the peripheral blood of the patients might be one parameter to evaluate patients’ prognosis.
ACKNOWLEDGEMENTS
The authors would like to thank all patients included in this study and staffs of Hematology Division for technical assisstance in the laboratory investigations. We also would like to thank Prof. Amnuay Thithapandha, Office of Academic Affairs, Faculty of Medicine Ramathibodi Hospital, Mahidol University for giving suggestions and improving the manuscript.
REFERENCES
1. Zon LI, Arkin C and Groopman JE. Haemato-logic manifestations of the human immune deficiency virus (HIV). Br J of Haematol 1987; 66: 251-6.
2. Scandden DT, Zon LI, Groopman JE. Pathophysiology and management of HIV-associated hematologic disorders. Blood 1989; 74: 145563.
3. Spivak JL, Selonick SE, Quinn TC. Acquired immune deficiency syndrome and pancytopenia. JAMA 1983; 250: 3084-7.
4. Franco CM, Hendrix LE, Lokey JL. Bone marrow abnormalities in the acquired immunodeficiency syndrome. Annals of Internal Medicine 1984; 101:275-6.
5. Osborne BM, Guarda LA, Butler J. Bone marrow biopsies in patients with the acquired immunodeficiency syndrome. Human Pathology 1984;15: 1048-53.
6. Spivak JL, Bender BS, Quinn TC. Haemato-logic abnormalities in the acquired immune deficiency syndrome. Am J Med 1984; 77: 224-8.
7. Moller T, Hasselbalch HC. Hematological changes associated with human immunodeficiency virus (HIV-1) infection. Ugeskr Laeger 1993; 155(19): 1442-6.
8. Schneider DR, Picker LJ. Myelodysplasia in the acquired immune deficiency syndrome. Am J Clin Pathol 1985; 84: 144-52.
9. Sivaram M, White A, Radcliffe KW. Eosino-philia: clinical significance in HIV-infected individuals. Int J STD AIDs 2012; 23(9): 635-8.
10. Harris PJ. Eosinophils and AIDS. Medical Hypotheses 1994; 43: 75-6.
11. Rothenberg ME. Eosinophilia. N Engl J Med 1998; 338: 1592-600.
12. Skiest DJ, Keiser P. Clinical significance of eosinophilia in HIV-infected individuals. Am J Med 1997; 102 (5): 449-53.
13. Tietz A, Sponagel L, Erb P, Bucher H, Batte-gay M, Zimmerli W. Eosinophilia in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 1997; 16(9): 675-7.
14. Cohen AJ, Steigbigel RT. Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis 1996; 174(3): 615-8.
15. Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm Rep 2001; 50 (RR-19): 1-57.
16. Mohandas N, Kim YR, Tycko DH, et al. Accurate and independent measurement of volume and hemoglobin concentration of individual red cells by laser light scattering. Blood 1986; 68: 506-13.
17. Bollinger PB, Drewinko B, Brailas CD, et al. The Technicon H*1TM automated hematology analyzer for today and tomorrow. Am J Clin Pathol 1987; 87: 71-9.
18. Dams E TH M, Mascart-Lemone F, Schandene L, Van Der Meer JWM. An unusual case of severe combined immunodeficiency with hy-poeosinophilia. J Int Med 1997; 242: 267-9.
19. Caterino-de-Araujo A. HIV-1 infection and eo-sinophilia. Immunology Today 1994; 15: 4989.
20. Elliott AM, Kyosiimire J, Quigley MA, Na-kiyingi J, Watera C, Brown M, et. Al. Eosino-philia and progression to active tuberculosis in HIV-1- infected Ugandans. Trans R Soc Trop Med Hyg 2003; 97: 477-80.
21. Sanderson CJ. Interleukin-5, Eosinophils, and Disease. Blood 1992; 79: 3101-9.
22. Bruggen vd T, Hoven CE, Kanters D et. al. Interleukin-5 signaling in human eosinophils involves JAK 2 Tyrosine Kinase and STAT 1a. Blood 1995; 85: 1442-8.
23. Riedl D, Lindemann A, Brach M, Mertelsmann R, Herrmann F. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce surface expression of interleukin-2 receptor p55- chain and CD4 by human eosinophils. Immunology 1990; 70: 258-60.
24. Kaminsky RG, Soto RJ, Campa A, Baum MK. Intestinal parasitic infections and eosinophilia in a human immunodeficiency virus positive population in Honduras. Mem Inst Oswaldo Cruz, Rio de Janeiro 2004; 99(7):773-8.
25. Cohen AJ, Steigbigel RT. Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis 1996; 174: 615-8.
26. Smith KJ, Skelton HG, Yeager J, Ledsky R, Ng TH, Wagner KF. Increased drug reactions in HIV-1-positive patients: a possible explanation based on patterns of immune dysregulation seen in HIV-1 disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). Clin Exp Dermatol 1997; 22: 118-23.


