Increases of pro-inflammatory cytokine expression in hippocampus following chronic paracetamol treatment in rats
Chattraporn Chantong1, Waranurin Yisarakun1, Thananya Thongtan2,
Supang Maneesri-le Grand1*
1 Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
2 Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
Email addresses: CC: chatter_ahs14@hotmail.com WY: waranurin@hotmail.com TT: tthongtan@yahoo.com
SMG: le.grand.maneesri.s@gmail.com
Corresponding author: Dr. Supang Maneesri-le Grand
Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok, 10330, Thailand
Telephone: +66 2 256 4000 ext. 3519
Facsimile: +66 2 252 7854
E-mail: legrand.maneesri.s@gmail.com
Received 20 August 2013; Accepted 8 October 2013
ABSTRACT
Background: Paracetamol is one of the most popular drugs used for treatment of pain and headaches. Recent evidences have indicated that long term treatment with this drug can induce the neurotoxic effect in the brain including in the hippocampal neurons, however the mechanism underlying its neurotoxic effect is still unclear. The present study aimed to investigate the effect of long term treatment with paracetamol on the expression of pro-inflammatory cytokines IL-1α and TNF-α in the hippocampus using immunohistochemistry.
Methods: Male Wistar rats (250-300 g) were divided into control and paracetamol treated groups. A single dose for 0 day and a single daily paracetamol (200 mg/kg BW, intraperitoneally) treatment for a period of 5, 15, and 30 days was injected into the paracetamol-treated group (n=5, each) whereas the control group received vehicle injections at the same volume. After finishing the treatment, all rats were humanely killed by injection of a sodium pentobarbital overdose (i.p.). The expression of pro-inflammatory cytokines (IL-1α/TNF-α) in hippocampus was studied by immunohistochemical techniques.
Results: The results showed that short-term exposure with paracetamol (0 and 5 days) had no effect on the expression of IL-1α. However, long-term paracetamol treatment for 15 and 30 days induced a significant increase in the expression of IL-1α in the hippocampus compared with those observed in the control group. The expression of TNF-α showed an overall similar pattern with the immunohistochemical study of IL-1α. Interestingly, when comparing the level of pro-inflammatory cytokine expression in the paracetamol treated group between15 and 30 days of treatment, the expression of IL-1α and TNF-α in the 30 days paracetamol treated group was higher.
Conclusions: The present study demonstrates that long-term exposure with paracetamol induces an increase in the expression of pro-inflammatory cytokines (both IL-1α and TNF-α) in hippocampus. Since the increase of pro-inflammatory cytokines is associated with the degeneration of hippocampal neurons, with our present results it could be concluded that the abnormality of learning and memory in patients with long term exposure of paracetamol possibly exists.
Keywords: Paracetamol, pro-inflammatory cytokines, IL-1α, TNF-α, hippocampus
INTRODUCTION
Paracetamol (acetaminophen, APAP) is one of the most popular drugs used for the treatment of pain and several kinds of headache. Its popularity comes from various properties of this drug including the low price, high availability without any requirement of prescription and low side ef-fects1-4. It has been considered as a safe drug since almost no-side effect has been reported when using within the therapeutic doses5. However during the last decade, few studies have reported non-beneficial effects of this drug on several systems including the central nervous system (CNS)6-8. It is known that paracetamol can easily cross the blood brain barrier (BBB) [9] therefore the impact of this drug treatment on brain cells cannot be avoided.
In 2012, Posadas et al. had demonstrated that injection of paracetamol even in the doses below those required to produce hepatotoxicity could induce the neuronal cell death via the NF-KB signaling pathway10. In addition, a recent study in rats with chronic treatment (6 weeks) of this drug in combination with alcohol intake resulted in the damage of hippocampal neurons11. It is known that the hippocampal neurons are neurons in CNS which play a major role in the memory function and learning12, therefore the discovery of Fakunle et al. indicates that treatment with this drug might increase the possibility of memory impairment and learning loss in chronic usage. However the mechanism underlying this neurotoxic effect is still unclear.
Increase of pro-inflammatory cytokine production in hippocampus is well accepted as a major mechanism underlying the neuronal damage in this brain area. This mechanism is involved in several pathologic conditions related with neurodegeneration including the Alzheimer’s disease.
Paracetamol is known as a member of the non-steroidal anti-inflammatory drug (NSAID) despite its weak anti-inflammatory property13. Accumulating evidences have revealed the dual effect of this drug in inflammatory processes14-16. It can act as both a pro- and anti-inflammatory drug depending on the dose of the treatment. However, during the last 10 years, several studies have demonstrated that the duration of the treatment should be another concern for the effects of the treatment with paracetamol10, 17-18. Thus, we hypothesized that a long-term treatment with paracetamol could possibly disturb the balance of pro-inflammatory cytokines production in the hippocampus.
This present study aimed to investigate the effects of long-term treatment of paracetamol (200 mg/kg BW) on the production of pro-inflammatory cytokines (IL-1α and TNF-α ) in the hippocampus of the rat’s brain at four different time points (0, 5,15 and 30 days) using immunohistochemistry.
MATERIAL AND METHODS
Animals:
Adult male Wistar rats (250-300 g) were obtained from the National Laboratory Animal Center (Mahidol University, Thailand). The rats were housed in stainless steel cages and maintained with normal rat food and water under controlled environmental conditions. All procedures and tests were approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University, Thailand (06/55).
Study designs:
The rats were divided into the control and paracetamol-treated groups (n=40, each). The rats were further subdivided into 4 subgroups for paracetamol treatment (n=10, each) as follows: (a) 0 day, (b) 5 days, (c) 15 days and (d) 30 days. A single dose of paracetamol (200 mg/kg of BW) was injected into the rat intraperitoneally (i.p.) at day 0 and repeatedly injected with a single daily dose for 5, 15 and 30 days. In the control group, the rats received the vehicle injections of normal saline at the same volume. The expression of pro-inflammatory cytokines IL-1α and TNF-α were determined by using immunohistochemistry.
Sample collection:
At the specific time points, the rats were humanely sacrificed by i.p. injection of a sodium pentobarbital overdose then perfused transcardially with 250 mL of phosphate buffer, followed by 250 mL of 4% paraformaldehyde in 0.1M phosphate buffer pH 7.4. Next, the brain was removed. The thick brain slices were cut at 3 mm anterior to bregma and then immersed in a 4% paraformaldehyde 0.1M phosphate buffer. After overnight fixation, the tissues were processed for paraffin embedding. These samples were processed for immunohistochemistry.
Immunohistochemical techniques:
Serial coronal brain sections were cut at 3 pm thickness by using a microtome, and collected 1 in 12 series. In total, 6 sections were collected from each animal. The sections were collected on the SuperFrost Plus Slide and heated overnight at 60°C before processing for deparaffinization. Brain sections were deparaffinised and incubated in citrate buffer (pH 6.6) (Dako retrieval solution). Endogenous peroxidase blocking was performed by incubation with 3% hydrogen peroxide in 50% methanol. After that, the sections were incubated with 5% normal horse serum. In order to identify the expression of pro-inflammation cytokines (IL-1α, TNF-α), the sections were incubated with primary antibody rabbit anti-IL-1α (Santa Cruz Biotechnology, USA) and anti-TNF-α (Santa Cruz Biotechnology, USA) at the dilution of 1:100 overnight. The sections were then incubated with secondary antibody with HRP-conjugated (Dako EnVision kits, Glostrup, Denmark). The product of immunohistochemical reaction was visualized using the 3, 3'-diaminobenzi-dine (DAB) system (OptiView DAB IHC Detection Kit) and counterstained with haematoxylin (Dako, Glostrup, Denmark). The sections were dehydrated in a graded series of ethanol. After that all sections were coverslipped with a permanent mounting medium (Permount).
Four brain sections were selected from each animal. Slides were scanned and digitized by using an Aperio ScanScope (Aperio Technologies Inc., Vista, CA). After that they were investigated and analyzed by using ImageScope v.12.0.0.5039 software (Aperio Technologies Inc.). The immunore-action was analyzed for positive pixel counts with an optimized algorithm for cytoplasm staining (Positive
Pixel Count v.9 parameters). Immunoreactivity of pro-inflammatory cytokines IL-1α and TNF-α are reported as % of positive pixel per square millimeters (pixel intensity/mm2). Each slide was evaluated by two independent observers who were blinded to the animal classifications.
Statistics
The results were expressed as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism v.6 software (GraphPad, San Diego, CA, USA) by two-way analysis of variance (ANOVA), followed by the Bonferroni post hoc test. All mean differences were considered to be statistically significant at p < 0.05.
RESULTS
Long-term treatment with paracetamol increases pro-inflammatory cytokines in hippocampus of the rat’s brain:
Interleukin-1 alpha (IL-1α)
The results from immunohistochemical study demonstrated that the paracetamol treatment for 0 and 5 days had no effect on the expression of IL-1α. However, the long-term treatment with paracetamol for 15 and 30 days significantly increased the IL-1α expression as compared with that of the control group. The data are shown in Figure 1. Interestingly, when comparing the level of IL-1α expression in the paracetamol treated group between 15 and 30 days, the expression of IL-1α in the 30 days paracetamol treated group was higher.
The results obtained from this part of the study indicate that short-term paracetamol treatment (0 and 5 days) has no effect on the expression of IL-1α while the long-term paracetamol treatment (15 and 30 days) can induce an increase in this cytokine in the hippocampus.
Tumor necrosis factor (TNF-α)
The immuno staining for TNF-α in the hippocampus are well correlated with those results from the expression of IL-1α. The expression of TNF-α was not affected by the short-term paracetamol treatment (0 and 5 days). Surprisingly, the long-term paracetamol treatment (15 and 30 days) could significantly increase the expression of TNF-α compared with those observed in the control group. The expression of TNF-α in the paracetamol treated group for 30 days was higher than that of 15 days treatment as demonstrated in Figure 2.
As the results from this study, the short-term paracetamol treatment (0 and 5 days) has no effect on the expression of TNF-α. In contrast, long-term paracetamol treatment (15 and 30 days) can induce a significant increase in the expression of TNF-α in the hippocampus compared with those observed in the control group.
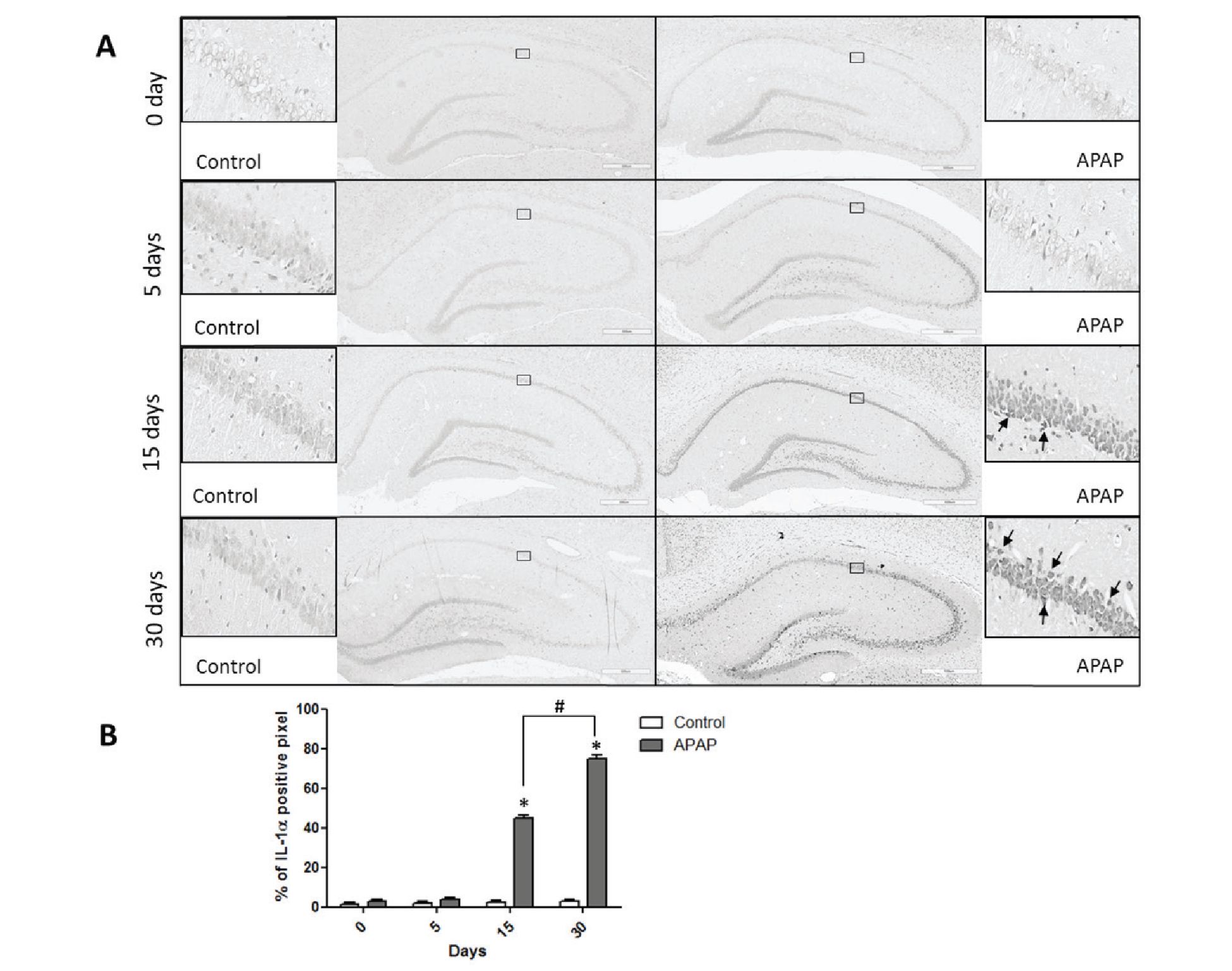
Figure 1 Effect of paracetamol treatment on the IL-1α expression in the hippocampus.
(A) Photomicrographs showing the expression of the IL-1α immunoreactive cells (arrow) in the hippocampus obtained from the control and paracetamol treated groups at four different time points (0, 5, 15, 30 days).
(B) Histogram showing the average percentage of IL-1α positive cells in the hippocampus obtained from the control and paracetamol treated groups at four different time points (0, 5, 15, 30 days). *p< 0.05 compared with the control group. #p< 0.05 compared with the paracetamol treated group at day 15.
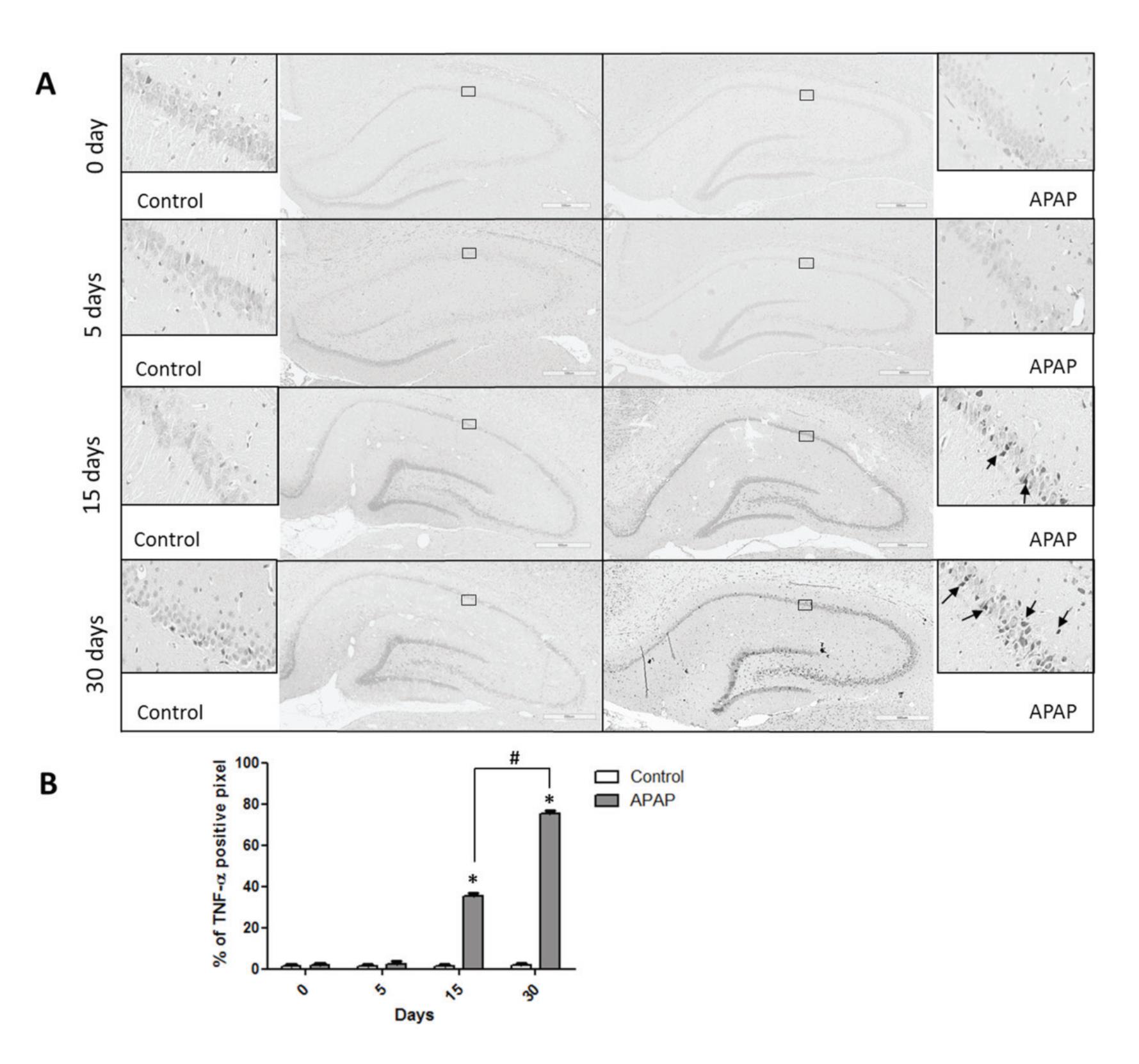
Figure 2 Effect of paracetamol treatment on the TNF-α expression in the hippocampus.
(A) Photomicrographs showing the expression of the TNF-α immunoreactive cells (arrow) in the hippocampus obtained from the control and paracetamol treated groups at four different time points (0, 5, 15, 30 days).
(B) Histogram showing the average percentage of TNF-α positive cells in the hippocampus obtained from the control and paracetamol treated groups at four different time points (0, 5, 15, 30 days). *p< 0.05 compared with the control group. #p< 0.05 compared with the paracetamol treated group at day 15.
DISCUSSION
The present data shows that short-term and long-term paracetamol treatment has a different effect on the expression of pro-inflammatory cytokines in hippocampus. Short-term treatments, at the periods of 0 and 5 days, have no effect on the expression of pro-inflammatory cytokines IL-1α and TNF-α in hippocampus. However, long-term treatment with this drug, treatments for 15 and 30 days, result in an increase in the IL-1α and TNF-α expression in the hippocampus. In addition, comparing between the long-term paracetamol treated groups of 15 and 30 days, the expression of these pro-inflammatory cytokines of the 30 days paracetamol treated group was higher than that of the 15-day treatment group.
In this study, we have found that the effect of paracetamol treatment on the alteration in hippocampus is not a secondary effect from the hepatotoxic effect of this drug since all three liver enzymes indicating the liver function from the rats with the paracetamol treatments (every period) were not different from the control group. In addition, the body weight of the animal with paracetamol treatment was not different from that of the control group indicating no difference of the food consumption between these two groups (data not shown). Based on these results, it can be suggested that all alterations observed in paracetamol treatment are direct effect of this drug on the hippocampus.
Concerning the effect of paracetamol treatment on the hippocampal neurons which are the neurons responsible for memory and learning function, several studies have demonstrated that this drug can provide either protective or non-beneficial actions on these neurons. Several in vitro studies have revealed that paracetamol treatment could protect neurons including hippocampal neurons from several pathologic conditions such as oxida-
tive stress, and activation by amyloid P-peptides19-21. The neuroprotective action of this drug is suggested to be mediated via the antioxidant property and anti-inflammatory action of this drug19-21. A recent study in rats by Blecharz-Klin et al. has demonstrated that long-term treatment with a low dose of paracetamol results in the alteration of neurotransmission in hippocampus and enhancement of the working memory23. However, unusual non-beneficial effects of paracetamol on the hippocampus were gradually reported11, 23. The study in rats by Chen and Bazan has revealed that paracetamol could reduce hippocampal long-term potential (LTP). This effect was suggested to be mediated via a presynaptic 5-HT2 receptor activation23. In 2011, Fakunle et al. demonstrated that paracetamol treatment for 6 weeks (in combination with alcohol intake) could result in the loss of neuronal cells in hippocampus11. In this study the increase in the pro-inflammatory cytokine production in hippocampus was evident in rats with long-term treatment with paracetamol. Since the increase in pro-inflammatory cytokine is well accepted as a major factor involved in the damage of neurons, thus the increase in the hippocampal expression of IL-1α and TNF-α observed in this study can be one mechanism underlying the neurotoxic effect of paracetamol in hippocampus.
Our data also demonstrated that short-term treatment with paracetamol do not have any effect on the pro-inflammatory cytokine production in the hippocampus. This result strongly suggests that paracetamol treatment is safe for short-term exposure; however, long-term treatment with this drug (more than 15 days treatment) demonstrated different outcomes since it could induce the pro-inflammatory cytokine expression (IL-1α and TNF-α) in this brain area.
Thus, our results provided the evidence that confirms the adverse effect of this drug regarding the duration of the treatment. This part of the result is in line with several evidences reported during the last 10 years. Many adverse effects of long-term treatment with paracetamol were gradually revealed on several systems including the CNS17, 24-25. It is known that the majority of this drug, after reaching the circulation, is mainly metabolized in the liver via conjugation with glucuronic, sulphate, or cysteine and then excreted into the urine as a non-toxic metabolite. Only a small fraction of this drug is metabolized by cytochrome P-450, resulting in the formation of n-acetyl-p-benzoquinone imine (NAPQI) [26-27]. The NAPQI is a toxic substance, after forming it is quickly captured by glutathione (GSH) and also excreted into the urine. The CYP2E1 is the most abundant isoform of cytochrome P-450 which is expressed in several organs including in the brain 17, 28-29. The presence of this enzyme in neurons, astrocyte, microglia and endothelial cells were reported through several studies6, 30-31. Although CYP2E1 in the brain is presented at low levels, its activity is actually higher than those observed in the liver32. In this case, after paracetamol reaches the cerebral circulation, it can pass through the BBB and be metabolized into NAPQI by those cells which express CYP2E1. As mention earlier, NAPQI is a toxic substance, thus it will be detoxified quickly by brain GSH. In case of a high concentration of paracetamol, the excessive level of NAPQI can either directly bind to cellular proteins leading to the cellular damage and death17, 27, 33-34 or indirectly induce the depletion of GSH in the brain. It is known that the depletion of GSH can result in the increase of oxidative stress which consequently alters the balance of pro-inflammatory cytokine production27. Therefore, we suggest that long-term treatment with paracetamol can lead to the increment of NAPQI formation as well as oxidative stress. These phenomenon can finally cause an increase in the pro-inflammatory cytokines expression in several regions of the brain including hippocampus.
In summary, the long-term treatment with paracetamol can induce an increment of pro-inflammatory cytokines expression in the hippocampus. The increase of NAPQI and oxidative stress are all involved in these alterations. However, the exact mechanism underlying an enhancement of pro-inflammatory cytokines production in hippocampus induced by long-term exposure with paracetamol still needs to be clarified. Since the increase in the pro-inflammatory cytokines can damage the neuronal cells, thus the increase of IL-1α and TNF-α expression in hippocampus following chronic treatment with paracetamol can indicate the significant impact in the long-term usage of this drug on the learning and memory function.
ACKNOWLEDGEMENTS
The authors gratefully acknowledged Professor Pichet Sampatanukul for his valuable comments and suggestions. This study was supported by the National Research Council of Thailand (NRCT, GRB_BSS_48_55_30_07) and Thailand Research Fund (RSA 5580034).
REFERENCES
1. Tripathy D, Grammas P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc Res 2009; 77: 289-296.
2. Smith HS. Potential Analgesic Mechanisms of Acetaminophen. Pain Physician 2009; 12: 269280.
3. Anderson B. Paracetamol (acetaminophen): mechanism of action. Pediatr Anesth 2008; 18: 915-921.
4. Graham GG, Scott KF. Mechanism of Action of Paracetamol. Am J Ther 2005; 12: 46-55.
5. Kurtovic J, Riordan SM. Paracetamol-induced hepatotoxicity at recommended dosage. J Intern Med 2003; 253: 240-243.
6. Posadas I, Santos P, Blanco A, Munoz-Fernan-dez M, Cena V. Acetaminophen Induces Apoptosis in Rat Cortical Neurons. PLoS ONE 2010; 5: e15360.
7. Srikiatkhachorn A, Tarasub N, Govitrapong P. Acetaminophen-induced antinociception via central 5-HT2A receptors. Neurochem Int 1999; 34: 491-498.
8. Supornsilpchai W, le Grand SM, Srikiatkhachorn A. Involvement of pro-nociceptive 5-HT2A receptor in the pathogenesis of medication-overuse headache. Headache 2010; 50:185-197.
9. Fischer LJ, Green MD, Harman AW. Levels of acetaminophen and its metabolites in mouse tissues after a toxic dose. J Pharmacol Exp Ther 1981; 219: 281-286.
10. Posadas I, Santos P, Cena V. Acetaminophen induces human neuroblastoma cell death through NF-kB activation. PLoS ONE 2012; 7: e50160.
11. Fakunle PB, Ajibade AJ, Oyewo EB, Alamu OA, Daramola AK. Neurohistological degeneration of the hippocampal formation following chronic simultaneous administration of ethanol and acetaminophen in adult Wistar rats (Rattus norvegicus). Journal of Pharmacology and Toxicology 2011; 6: 701-709.
12. Rolls ET. A theory of hippocampal function in memory. Hippocampus 1996; 6: 601-620.
13. Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin Infect Dis 2000; 31 Suppl 5: S202-210.
14. Dambach DM, Durham SK, Laskin JD, Laskin DL. Distinct roles of NF-kappa B p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol Appl Pharm 2006; 211: 157-165.
15. Gardner CR, Laskin JD, Dambach DM, et al. Exaggerated hepatoxicity of acetaminophen in mice lack tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol 2003; 192: 118-130.
16. Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol 2007; 20: 734-44.
17. James LP, Mayeux PR, Hinson J.A. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 2003; 31: 1499-1506.
18. Van der Kraan PM, Vitters EL, De Vries BJ, van den Berg WB, van de Putte L.B. The effect of chronic paracetamol administration to rats on the glycosaminoglycan content of patellar cartilage. Agent and Actions 1990; 29: 218-223.
19. Bisaglia M, Venezia V, Piccioli P, et al. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int 2002; 41: 43-54.
20. Landolfi C, Soldo L, Polenzani L, et al. Inflammatory molecule release by beta-amyloid-treated T98G astrocytoma cells: role of prostaglandins and modulation by paracetamol. Eur J Pharmacol. 1998; 360: 55-64.
21. Tripathy D, Grammas P. Acetaminophen inhibits neuronal inflammation and protects neurons oxidative stress. J Neuroinflamm 2009; 6: 1-9.
22. Blecharz-Klin K, Piechal A, Pyrzanowska J, Jo-niec-Maciejak I, Kiliszek P, Widy-Tyszkiewicz E. Paracetamol-The outcome on neurotransmission and spatial learning in rats. Behav Brain Res 2013; 253: 157-164.
23. Chen C, Bazan NG. Acetaminophen modifies hippocampal synaptic plasticity via a presynaptic 5-HT2 receptor. Neuroreport 2003; 14: 743747.
24. Hasler J.A. Human cytochromes P450. Mol Aspects Med 1999; 20: 1-137.
25. Ward B, Alexander-Williams JM. Paracetamol revisited: A review of the pharmacokinetics and pharmacodynamics. Acute Pain 1999; 2: 139149.
26. Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol 2008; 11: 379-395.
27. Parson AA, Strijbos P. The neuronal versus vascular hypothesis of migraine and cortical spreading depression. Curr Opin Pharmacol 2003; 3:73-77.
28. Hansen AJ, Zeuthen T. Extracellular ion concentration during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand 1981; 113: 437-445.
29. Davies J.A, Annels SJ, Dickie BG, Ellis Y, Knott NJ. A comparison between the stimulated and paroxysmal release of endogenous amino acids from rat cerebellar, striatal and hippocampal slices: a manifestation of spreading depression? J Neurol Sci 1995; 131: 8-14.
30. Hansson T, Tindberg N, Ingelman-Sundberg M, Kohler C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience 1990; 34: 451463.
31. Haorah J, Knipe B, Leibhart J, Ghorpade A, Persidsky Y. Alcohol-induced oxidative stress in brain endothelial cells causes blood-brain barrier dysfunction. J Leukoc Biol 2005; 78: 1223-1232.
32. Mayevsky A, Weiss HR. Cerebral blood flow and oxygen consumption in cortical spreading depression. J Cereb Blood Flow Metab 1991; 11: 829-836.
33. Jander S, Schroeter M, Peters O, Witte OW, Stoll, G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow 2001; 21: 218225.
34. Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 1993; 43: S16-S20.


