Contribution of MYC FISH in Pathological Diagnosis of Burkitt Lymphoma
Ubon Poumsuk1, Thiamjit Chaichana1, Preecha Ruangvejvorachai1, Thamathorn Assanasen1
and Shanop Shuangshoti12
1Department of Pathology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, THAILAND
2Chulalongkorn GenePRO Center, Research Affairs, Faculty of Medicine, Chulalongkorn University,
Bangkok 10330, THAILAND
Correspondence: Shanop Shuangshoti, MD
E-mail: Shanop@gmail.com Tel: +662 256 4235 Fax: +662 652 4208
Received: 10 April 15; Accepted 5 June 15
ABSTRACT
Burkitt lymphoma (BL) is an aggressive B-cell non-Hodgkin lymphoma typically with MYC rearrangement. Distinction between BL and diffuse large B cell lymphoma (DLBCL) is not infrequently encountered in routine practice. In this communication, we report our experience with the MYC break-apart probe FISH in the diagnosis of BL in 19 cases of aggressive B-cell lymphoma. BL was the final diagnosis in 10/19 cases (52.63%), and 9 of them were shown to carry MYC gene rearrangement. DLBCL was the final diagnosis in 7 cases and all had negative FISH test. B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL were diagnosed in the remaining 2 cases, and one of them showed MYC rearrangement. To summarize, identification of MYC gene rearrangement by FISH assay helps supporting the diagnosis of BL in most of the cases.
Keywords: Burkitt Lymphoma, Diffuse large B cell lymphoma, MYC FISH
Running title: MYC FISH in Diagnosis of Burkitt Lymphoma
INTRODUCTION
Burkitt lymphoma (BL) was first described by Denis Burkitt in 19581. It is an aggressive non- Hodgkin lymphoma of B-cell lineage. Since the original description, tremendous progress has been made in understanding molecular mechanisms of the cancer. Pathological diagnostic criteria have been changed and it is now clear that traditional morphological approach alone is not sufficient to make a firm diagnosis of BL.
One of the fairly common differential diagnoses in routine pathological practice is to distinguish between BL and diffuse large B cell lymphoma (DLBCL). Since dysregulation of MYC gene, mostly commonly t(8; 14) (q24;q32), features all cases of BL, assessment of the gene is diagnostically helpful2. In this communication, we report our experience with the MYC break-apart probe FISH in the diagnosis of BL.
MATERIALS AND METHODS
19 consecutive cases of malignant lymphoma underwent MYC break-apart probe FISH assay were retrieved from the pathological file at Department of Pathology, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Bangkok, during 2009 to 2012.
For all cases, the histologic differential diagnosis was between DLBCL and BL. The demographic data (age, gender, and site of lesion), immunophenotype, and MYC FISH result were recorded. Materials used in all cases were formalin-fixed paraffin-embedded tissue. Details of the antibodies used were shown in Table 1. MYC FISH was performed by break-apart (split signal) DNA probe (DAKO, Code Y5410). Translocation involving the MYC gene was scored when a specific signal pattern (co-localization of one pair of signals and segregation of the other, see Figure 1) was identified by a non-numerical approach3. Final diagnosis was rendered by combining the FISH result and other parameters.
Table 1 Details of primary antibodies and probe used.
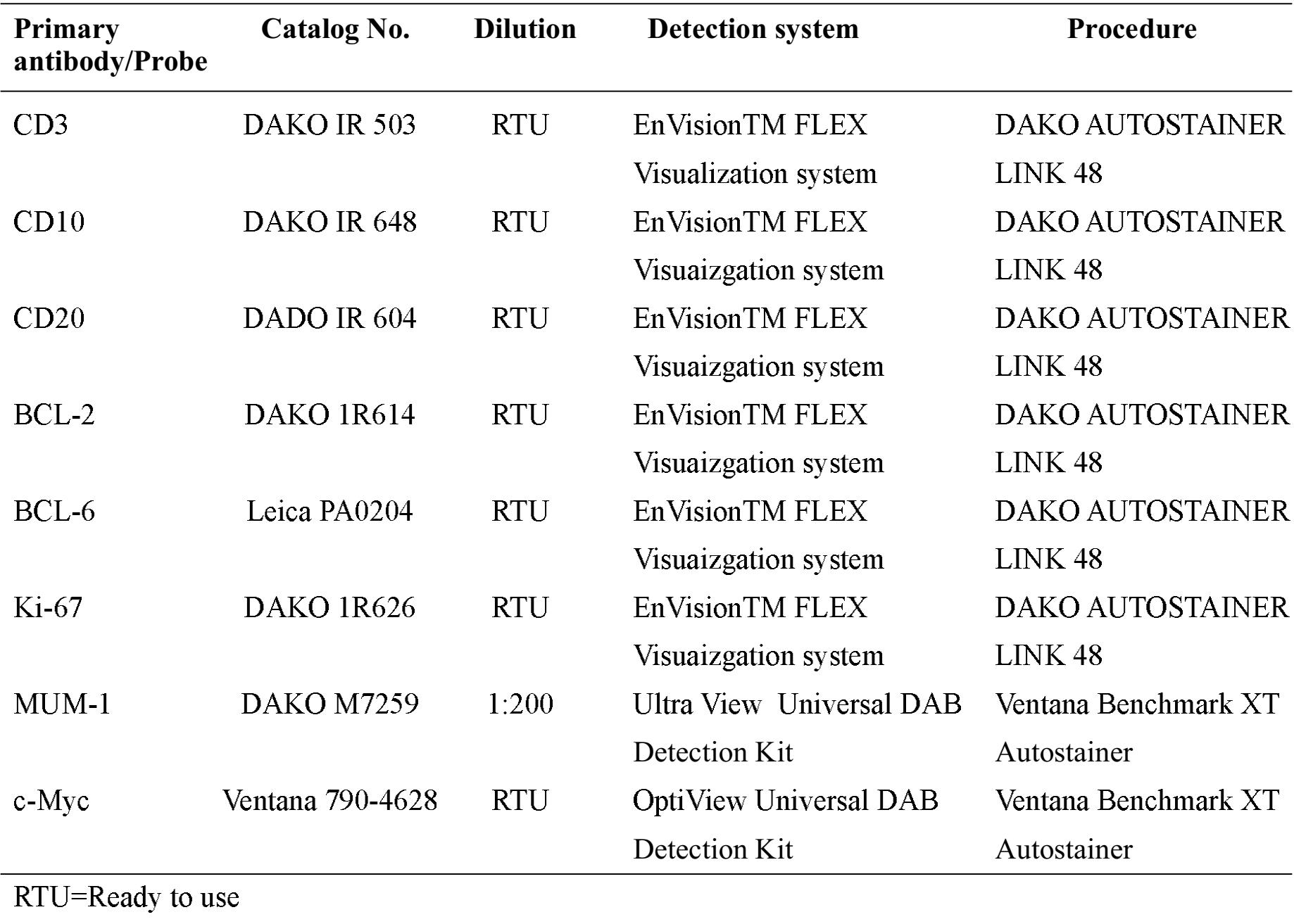

Figure 1 Diagram showing MYC gene evaluation by break-apart (split signal) DNA probes. The human MYC gene consists of 3 exons spanning a region of ~5 kb on chromosome 8 band q24. The FISH DNA probes are a mixture of a Texas Red-labeled DNA probe (MFC-Downstream) covering 418 kb telomeric to the MFC breakpoint cluster region (O), and a fluorescein-labeled DNA probe (MFC-Upstream) covering 652 kb centromeric to the MFC breakpoint cluster region (X). In normal cells, two fused signals are noted (left cell in figure). In cells with MFC gene rearrangement, 1 fused and 2 split signals are seen (right cell).
RESULTS
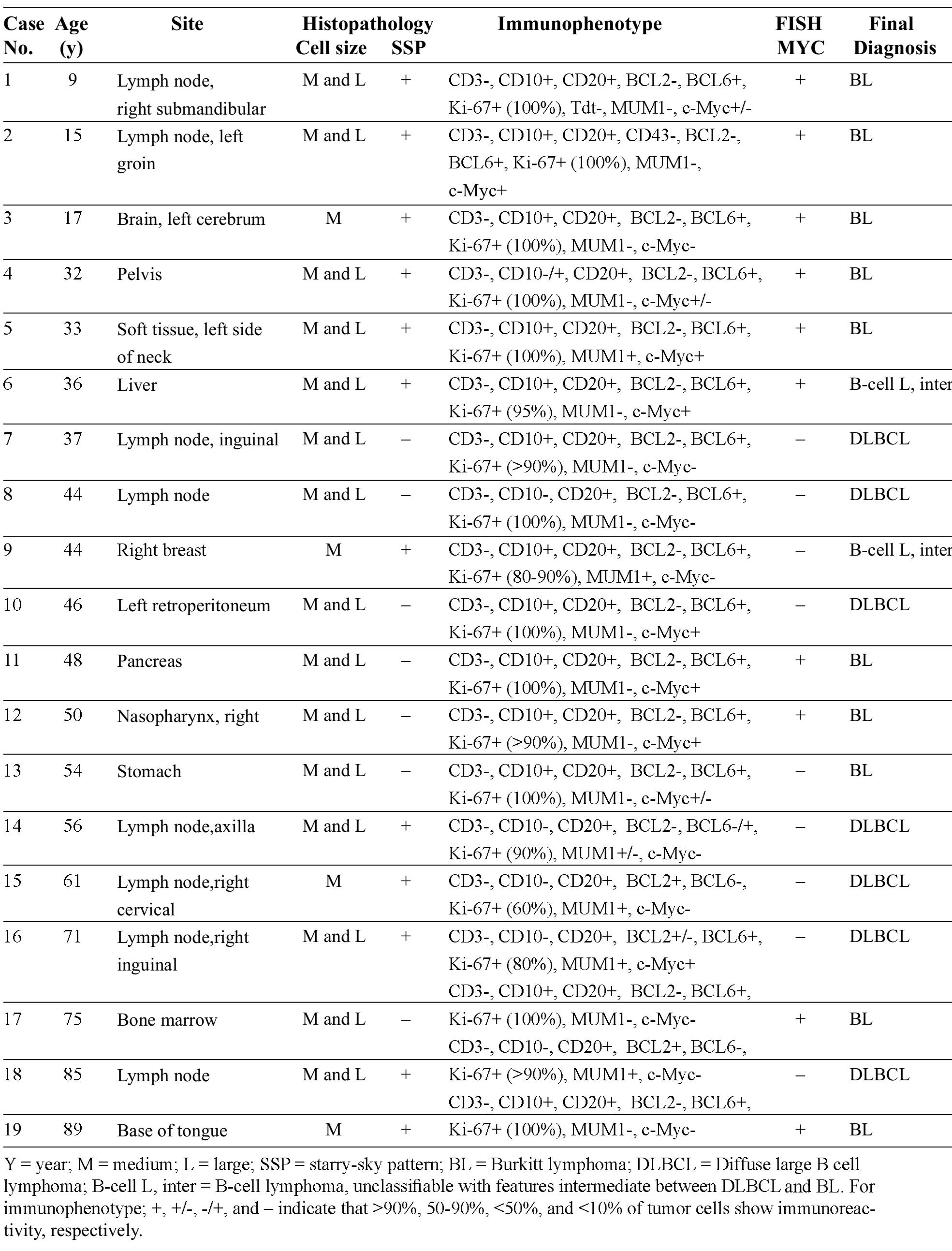
The patients’ age ranged from 9-89 years (mean = 47.47). There were 5 females and 14 males. Anatomical site, histopathology (nuclear size and starry-sky pattern), immunophenotype, MYC FISH result, and the final diagnosis of all cases were shown in Table 2. One BL (case # 19) tested for EBV showed positive result of EBER in situ hybridization.
Table 2 Details of cases and results
DISCUSSION
Distinction between diffuse large B cell lymphoma (DLBCL) and Burkitt lymphoma (BL) is a fairly common differential diagnosis in daily practice, and it is crucial since the latter requires more aggressive treatment. BL is high grade B-cell lymphoma that is characterized histologically by monomorphic proliferation of medium-sized non-cleaved malignant cells, with round nuclei, clumped chromatin, two to five nucleoli, medium-sized paracentral nucleoli, cytoplasmic vacuoles, and an appreciable rim of basophilic cytoplasm. Nuclear and cell membrane of the individual tumor cells frequently show molding or “Square-off’. High mitotic rate with “starry-sky” pattern imparted by tangible-body macrophages is characteristics, but not pathognomonic. DLBCL, despite the name implies (“large” cell), the size of tumor cells may be overlapped with that of BL. Furthermore, starry-sky pattern could also be encountered in DLBCL2.
Diagnosis of BL is currently based on several parameters, including histomorphology, immunophenotype, cytogenetic, molecular study, and gene expression profiles; since there is no single test that can be used as gold standard for the diagnosis4. Immunohistochemical study is helpful, to some extent, in the differential diagnosis between DLBCL and BL, and the typical profile is summarized in Table 3.
Table 3 Immunophenotype of DLBCL and BL2 DLBCL BL
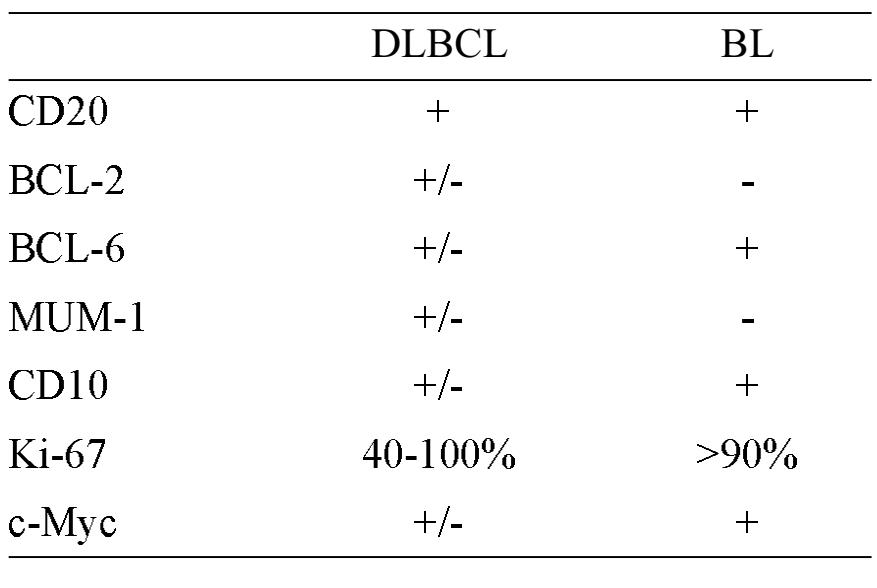
Despite the IHC panel, diagnosis can still be ambiguous, and assessment of MYC gene rearrangement is needed. Having said that, translocation involving MYC gene, though characteristic, is not specific for BL. Furthermore, diagnosis of BL can still be made when MYC FISH test is negative if all other features (morphology, CD20+, CD10+, BCL6+, BCL2-, and Ki67+ >95%) fit well with the diagnosis 2, 5.
In 2008, a new category of B cell neoplasm, “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL” was introduced by the World Health Organization2. Diagnostic criteria of the entity include (1) morphologic overlap between DLBCL and BL with typical im-munophenotype of BL, (2) morphologic features of BL with atypical immunophenotype, and (3) MYC rearrangement with concomitant BCL2 and/or BCL6 rearrangement (so called double-hit lymphomas)2,6.
In our cohort of 19 B-cell lymphomas with the differential diagnosis between DLBCL and BL, positive MYC FISH supported the diagnosis of BL in 9 out of 10 cases. For the remaining BL case (#13) where FISH was negative, the diagnosis was based on clinical data, histomorphology and immunohis-tochemistry (IHC). 7/19 patients were diagnosed as having DLBCL and all of these tumors showed negative result of MYC FISH. It is important to note that discrepancy between c-Myc IHC and MYC FISH was found in 6/19 cases (31.58%): 3 cases with positive IHC but negative FISH (#10, 13, 16), and the others with the opposite result (#3, 17, 19). Mechanisms other than gene rearrangement may underlie the positive IHC in the former 3 cases whereas antigen loss may have resulted in the negative IHC (positive FISH) in the latter. B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL were diagnosed in 2 cases (cases # 6 and 9). The former case had overlapping morphological features between DLBCL and BL but typical immunophenotype of BL and positive FISH result; the latter showed typical BL morphology but atypical immunoprofile and no MYC rearrangement.
In summary, several parameters are required for the distinction between diffuse large B cell lymphoma (DLBCL) and Burkitt lymphoma (BL). In addition to morphology and immunoprofile, identification of MYC gene rearrangement by Ff SH assay helps supporting the diagnosis of BL in most cases.
REFERENCES
1. Coakley D. Denis Burkitt and his contribution to hematology/oncology. Br J Hematol 2006; 135: 17-25.
2. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (Eds.). WHO classification of tumors of hematopoietic and lymphoid tissues. Lyon: IARC; 2008: 233-235 and 262-266.
3. Haralambieva E, Banham AH, Bastard C, Del-sol G, Gaulard P, Ott G, Pileri S, Fletcher JA, Mason DY. Detection by the fluorescence in situ hybridization technique of MYC translocations in paraffin-embedded lymphoma biopsy samples. Br J Hematol 2003; 121:49-56.
4. Said J, Lones M, Yea S. Burkitt lymphoma and MYC: what else is new? Adv Anat PathoL 2014; 21:160-165.
5. Leucci E, Cocco M, Onnis A, De Falco G, van Cleef P, Bellan C, van Rijk A, Nyagol J, Byaki-ka B, Lazzi S, Tosi P, van Krieken H, Leoncini L. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J Pathol 2008; 216: 440-450.
6. Montgomery ND, Fedoriw Y. Pathology consultation on intermediate-to-large B-cell lymphomas. Am J ClinPathol 2014; 141: 305-317.


