Predictive Biomarker Testing in Colorectal Cancer: KRAS and BRAF Mutation Frequency and Testing Algorithm in Developing Countries
Niraj Kumari *, Narendra Krishnani **, AshokKumar***, Balraj Mittal ****
* Additional Professor, Department of Pathology
Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow
Email: nirajpath@yahoo.co.in. Fax: 91 522 2668017, Phone: 91 522 2495236
** Professor, Department of Pathology Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow
Email: narendrakrishnani@yahoo.co.in, Fax: 91 522 2668017, Phone: 91 522 2494246
*** Professor, Department of Surgical Gastroenterology Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow
Email: doc.ashokgupta@gmail.com, Fax: 91 522 2668017, Phone: 91 522 2494324
**** Professor, Department of Biotechnology Baba Saheb Ambedkar University Lucknow, India
Email: ba.1rajmitt.a.1@gma.il.com., Fax: 91 522 2668017, Phone: 91 522 2494324
Correspondence: Niraj Kumari*
Received 12 november 2016; accepted 5 May 2017.
Abstract
Background: Predictive biomarker testing is mandatory before initiating targeted therapy in metastatic colorectal cancer (mCRC). Mutation frequencies and their association with clinicopathological features vary widely across populations. In low socio-economic countries, appropriate selection of cases is important for biomarker testing.
Methods: 110 CRC cases were evaluated for KRAS-BRAF mutation and association with clinicopathological features by PCR-Sanger sequencing method.
Results: Patients <40 years constituted 20%. Right and left-sided CRC constituted 34.5% and 63.5% respectively. KRAS mutation was seen in 18.2% and BRAF (V600E) in 0%. No association was found between KRAS mutation and clinicopathological features. All mCRC cases were wild except one. Patients aged <40 years showed lower mutation rate (13.6%) than patients of >40 years (21.6%). Young age was significantly associated with perineural invasion and necrosis. Right-sided tumors were significantly larger (>4 cm) than left-sided, had lower stage and lymphnode metastasis.
Conclusion: Lower KRAS mutation was found in CRC in north Indian patients. Young age and location but not mutation status showed significant association with tumor size, perineural invasion, necrosis, stage and lymphnode metastasis. In countries with low socio-economic conditions, predictive biomarker testing should be undertaken judiciously, starting with common biomarker testing followed by extended panel.
Running Title: Predictive Biomarker Testing And Colorectal Cancer
Keywords: metastatic colorectal cancer, KRAS, BRAF, predictive biomarker, anti-EGFR
Introduction
Colorectal cancer (CRC) is the third most common cancer in males and second most common cancer in females worldwide, however its frequency is quite low in the south Asian countries [1]. In Indian registries, incidence of rectal cancer is reported to be higher than colon cancer [2,3]. The localized CRC without lymph nodal metastasis may be cured by surgery alone, however the prognosis of metastatic disease is worse even after adjuvant chemotherapy or radiotherapy due to relapse of the disease in approximately 34% cases [4] which raises concern for development of more specific and targeted therapies to prevent relapse of disease in advanced cases of CRC. Monoclonal antibodies like cetuximab and panitumumab [5,6] are being currently used to target EGFR especially in metastatic CRC. The response of anti-EGFR therapy is determined by mutation status of downstream biomarkers (KRAS, BRAF) that influence cell proliferation, angiogenesis and migration [7-10]. CRC with wild type KRAS are believed to respond to anti-EGFR therapies, however 40-60% of patients with wild-type KRAS tumors do not respond to such therapy [11]. In these patients, data suggest that the mutated BRAF gene, which is present in 5-10% of tumors, can affect response to these agents [12-14]. Both the costs and potential toxicities associated with this management paradigm adds to the relevance of efforts to more critically define particular patient populations that would be most likely to respond to treatment with this class of agents, or, conversely, that would be highly unlikely to exhibit clinical benefit.
The mutation frequencies of various oncogenes and molecular biomarkers vary widely in different regions and in different populations. This study was planned with an aim to evaluate the mutation status of KRAS exon 1 and BRAF exon 15 in CRC and its correlation with clinicopathological parameters and followup in north Indian population.
Materials and Methods
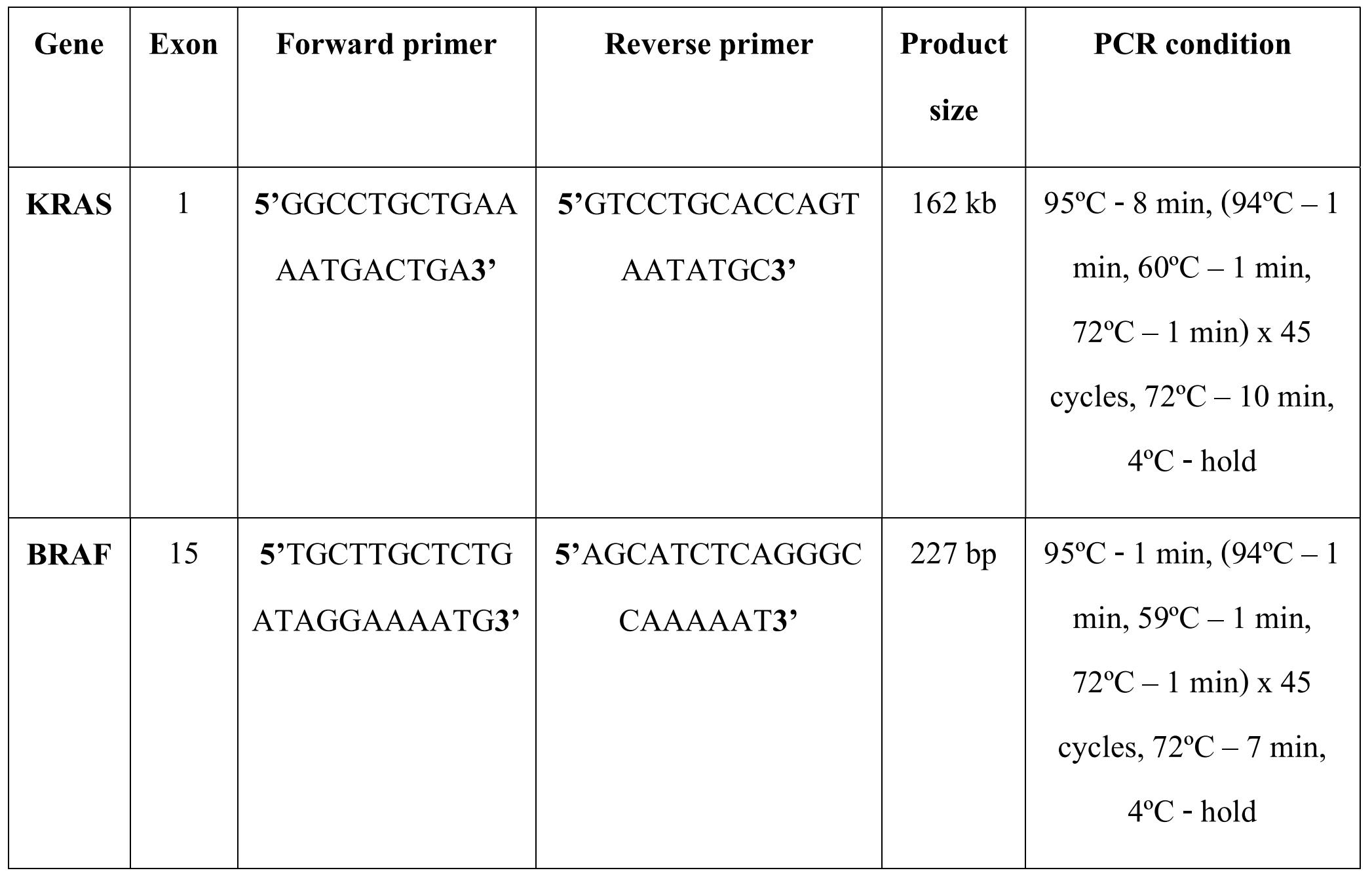
Consecutive cases of CRCs received within a period of 3 years in the Department of Pathology were included for this study. All the cases were reviewed for gross (size, location) and microscopic features (tumor differentiation, extent of infiltration, perineural and lymphovascular invasion, necrosis, presence of tumor infiltrating lymphocytes, mucinous areas and lymph node and distant metastases). The DNA was extracted from formalin fixed paraffin embedded tissues containing >90% tumor area. QiaAmp FFPE DNA extraction kit was used for DNA extraction. Mutation analysis for KRAS exon 2 and BRAF exon 15 was done by PCR-Sanger sequencing. The primer sequences and PCR cycles used in the study are given in table 1. Follow-up data was retrieved from the case files and Hospital Information System.
Table 1. Primer sequences and PCR conditions
Statistical Analysis
Chi square was used for analyzing categorical variables and Kaplan Meier log rank test was used for survival analysis.
Results
One hundred and ten consecutive cases of CRC were evaluated with M:F ratio of 2.5:1 (79 men, 31 women) having an age range of 23 years to 86 years (median 51 years, mean 52.5 years). Twenty-two patients (20%) were of age less than 40 years. The predominant histological type was conventional adenocarcinoma (92 cases) followed by mucinous and signet ring cell adenocarcinoma (16 cases) and papillary adenocarcinoma (2 cases). Proximal (right sided colon) CRCs accounted for 38 cases (34.5 %) and distal (left sided colon and rectum) accounted for 72 cases (63.5%), however, equal distribution was seen in colonic and rectal locations with each site accounting for 55 cases. KRAS mutation was detected in 20 (18.2%) cases. KRAS codon 12 mutations were seen in 12 (65%) cases and codon 13 in 7 (35%) cases (Figure 1a, 1b, 1c). Three different mutations were seen in codon 12. The commonest one was G12A, which was found in 9 cases followed by G12V in 3 cases and one case showed G12S. All 7 cases of codon 13 mutations were of G13A. BRAF V600E was not seen in any case, however two cases (1.8%) showed R603L and L588P mutations (Figure 2a, 2b). Two other cases showed single nucleotide polymorphisms (SNPs) at Q609Q and S602S and both these cases had mutation in KRAS gene. KRAS and BRAF mutation was seen equally in both proximal and distal CRCs, however KRAS mutation was marginally higher in colonic CRCs (12 cases, 60%) than rectal CRCs (8 cases, 40%).
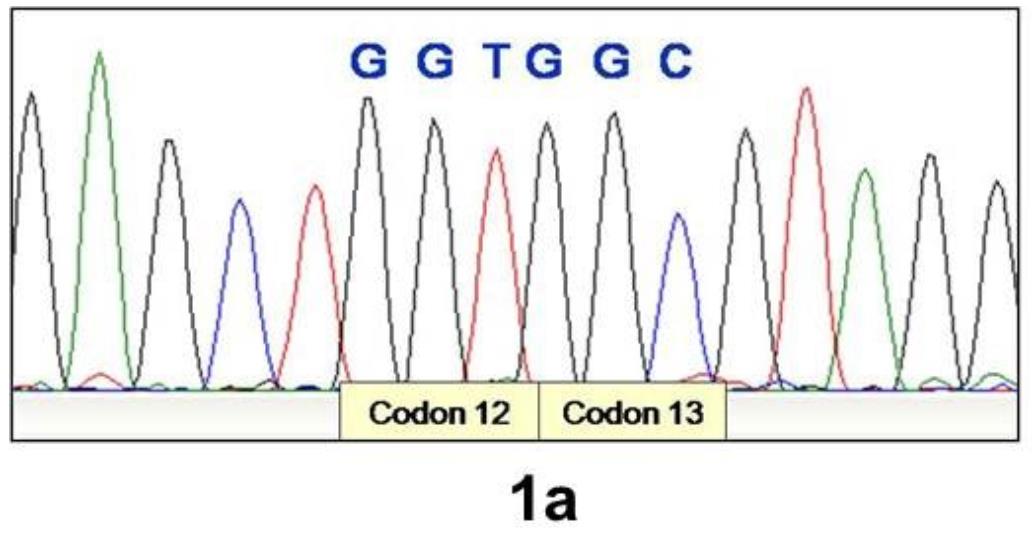
Figure 1a. Electrophoretogram showing wild type sequence of KRAS codon 12 and 13
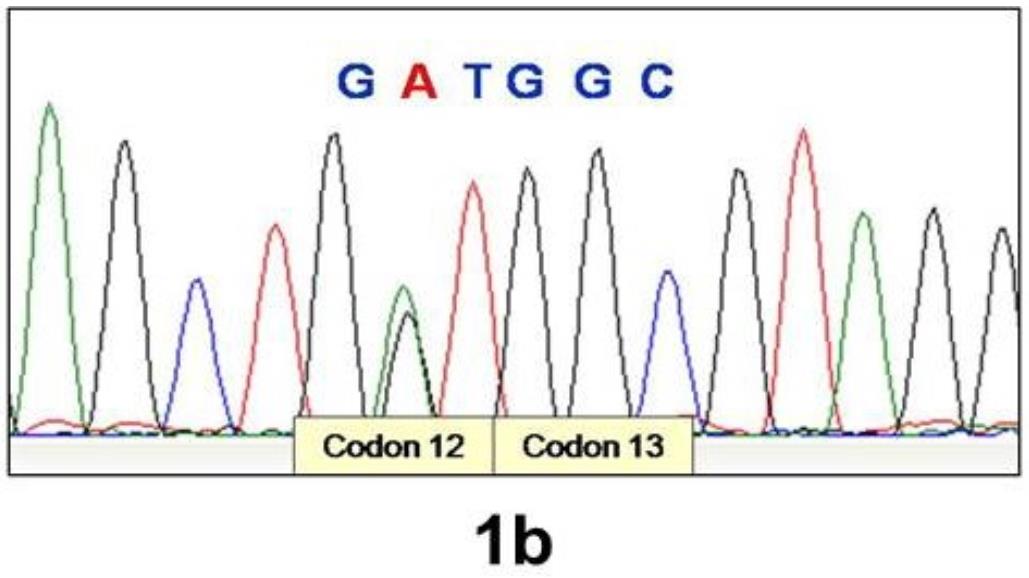
Figure 1b. Electrophoretogram showing G12A substitution in KRAS
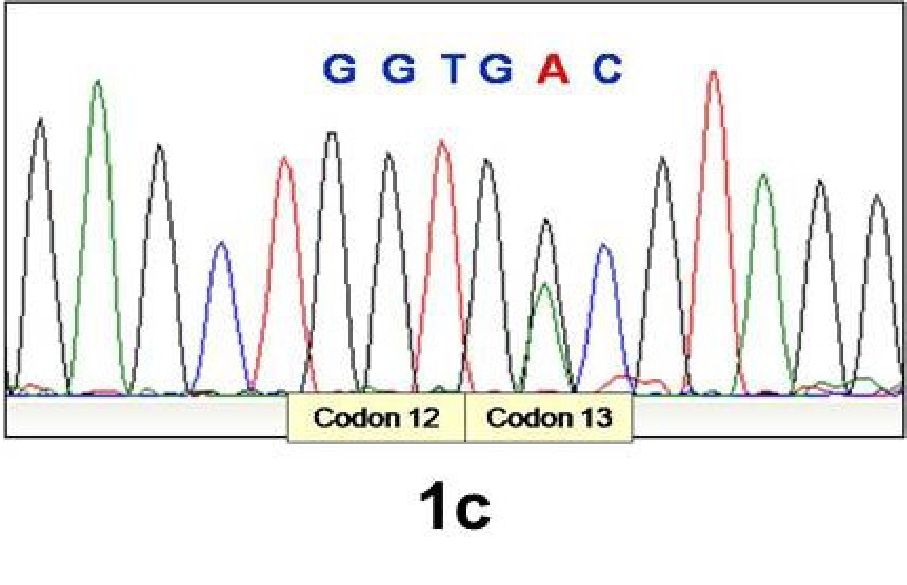
Figure 1c. Electrophoretogram showing G13A substitution in KRAS
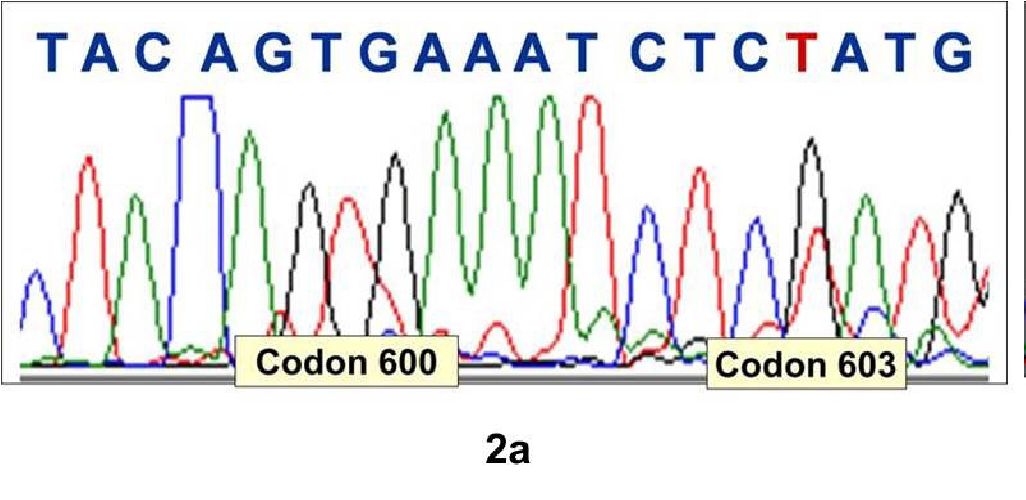
Figure 2a. Electrophoretogram showing wild type codon 600 and G603T substitution in BRAF
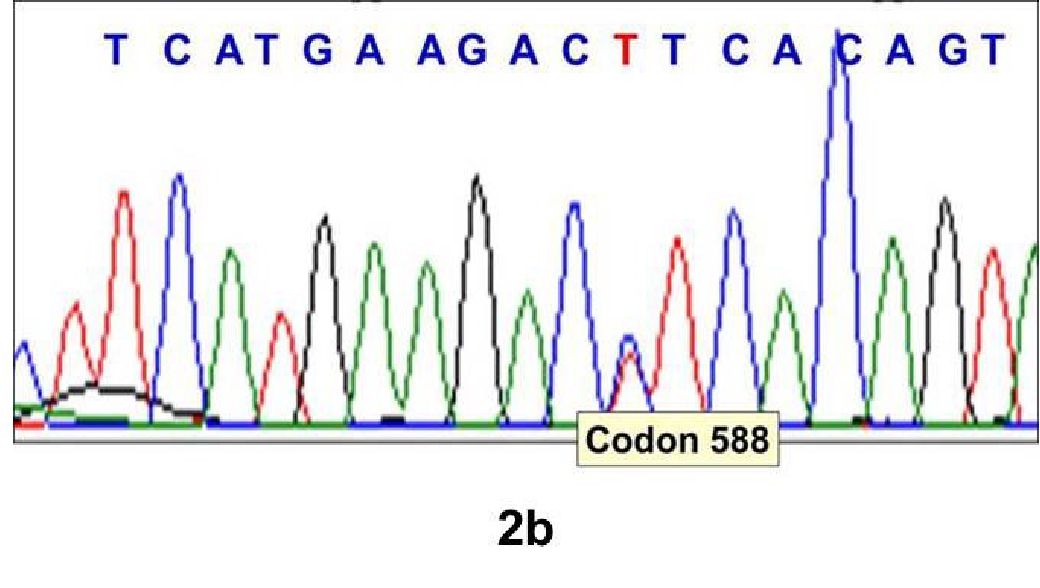
Figure 2b. Electrophoretogram showing C588T substitution in BRAF
There was no significant association between KRAS mutation and clinico-pathological features of CRC, however in BRAF though only two cases showed mutation. There were six cases that showed distant metastasis of which 5 were KRAS wild type and one was BRAF mutant. The correlation of KRAS and BRAF genotype with clinico-pathological features in CRC is given in table 2. The type of KRAS mutation in different codons also did not show any correlation with clinicopathological features (table 3).
Twenty percent cases (22 patients) with CRC were aged 40 years or younger. Three of the 22 patients aged 40 years and younger (13.6%) showed mutations with two cases (9.1%) harboring KRAS mutation and one case (4.5%) harboring BRAF mutation. In the age group of more than 40 years, 19 cases (21.6%) showed mutation with 18 cases (81.8%) harboring KRAS and one case (1.1%) harboring BRAF mutation. Younger age (<40 years) at presentation was significantly associated with perineural invasion (p=0.04) and necrosis (p=0.04) whereas distant metastasis showed a p value of 0.05. In both the age groups proximal colonic CRCs were more common than distal CRC, however younger patients had more of rectal involvement whereas patients aged more than 40 years had more colonic involvement (table 4).
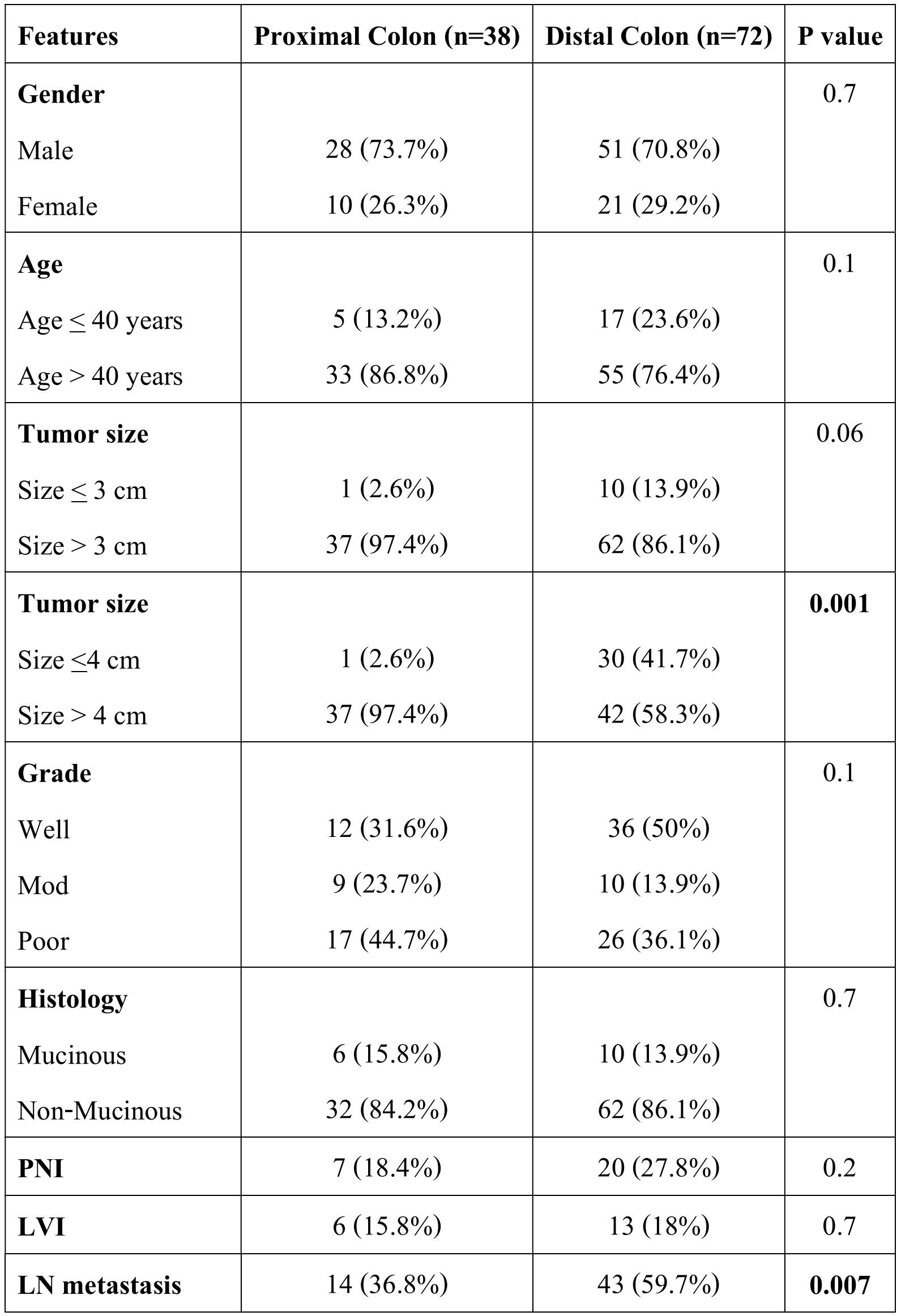
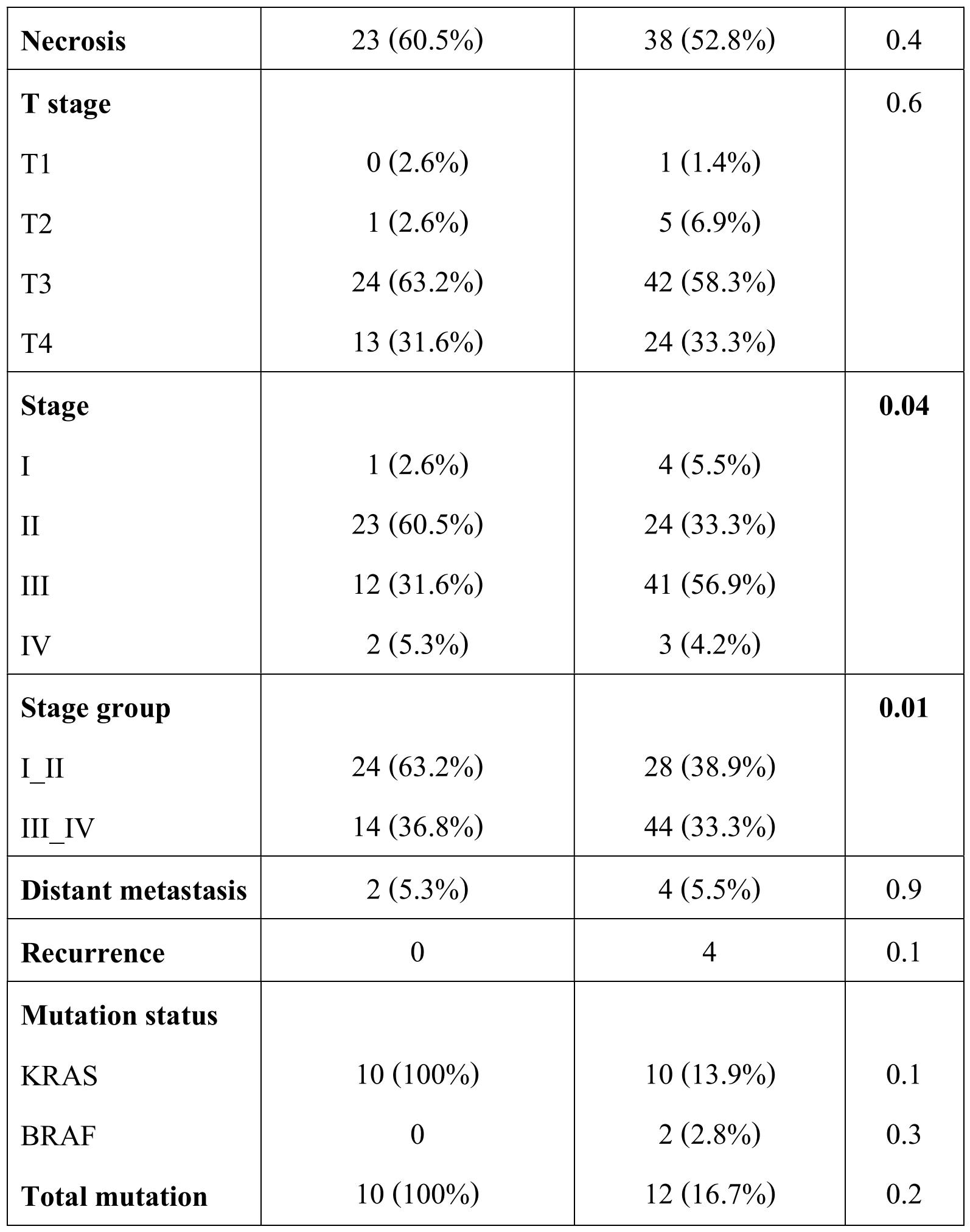
Anatomic location was also analyzed with clinicopathological features and mutation status. Proximal or right-sided colonic tumors showed association with larger tumor size (>4 cm) (p=0.001), low disease stage (p=0.04) and lower lymph node metastasis (p=0.007) (table 5).
Followup data was available in 94 cases ranging from 1 months to 96 months of which there were three deaths. The mean and median survival in KRAS mutant cases was 59 and 30 months respectively while in KRAS wild type mean and median survival was 87 and 19 months however, the difference was not statistically significant (p=-0.6).
Table 2: Correlation of clinicopathological features with KRAS - BRAF mutation status
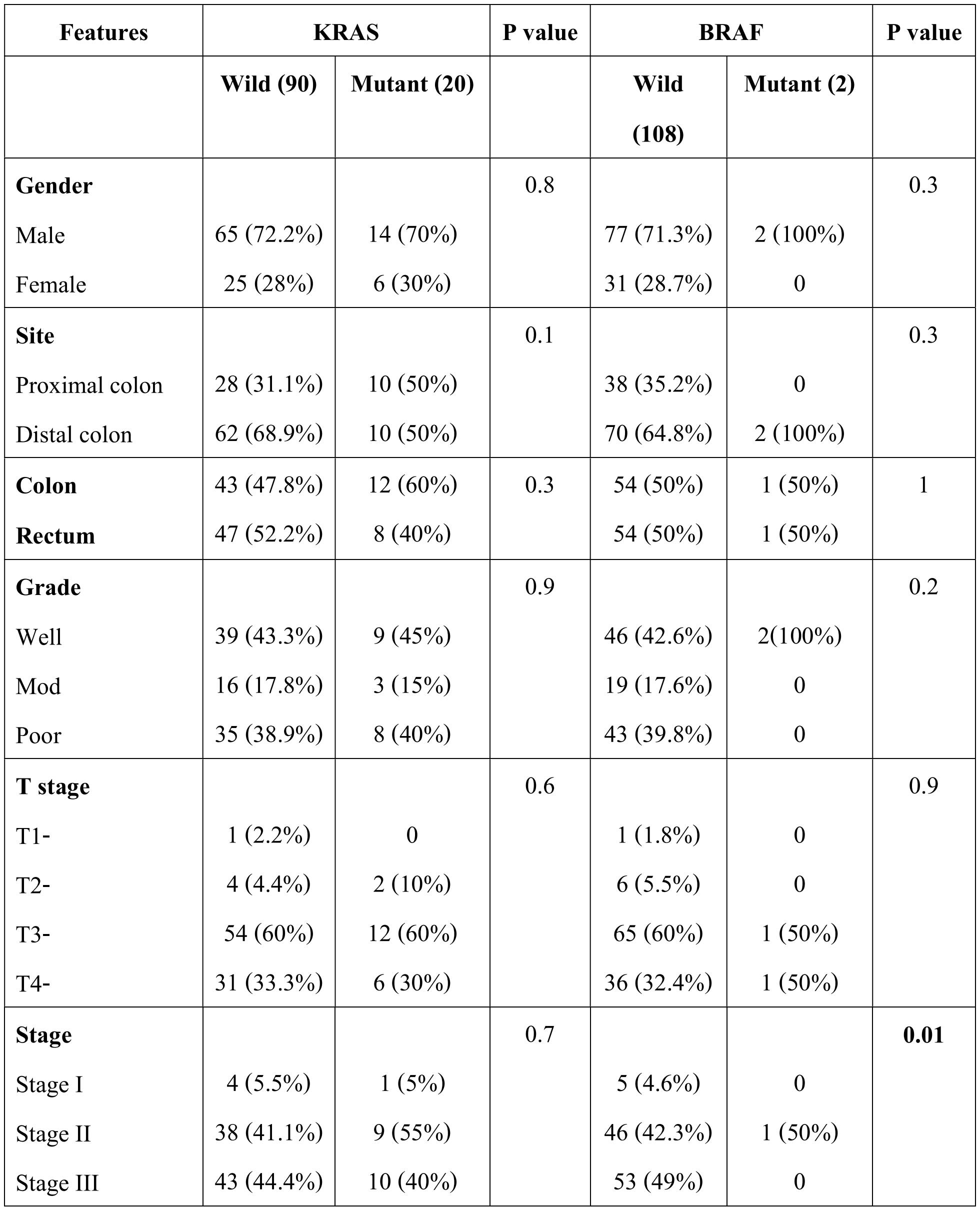
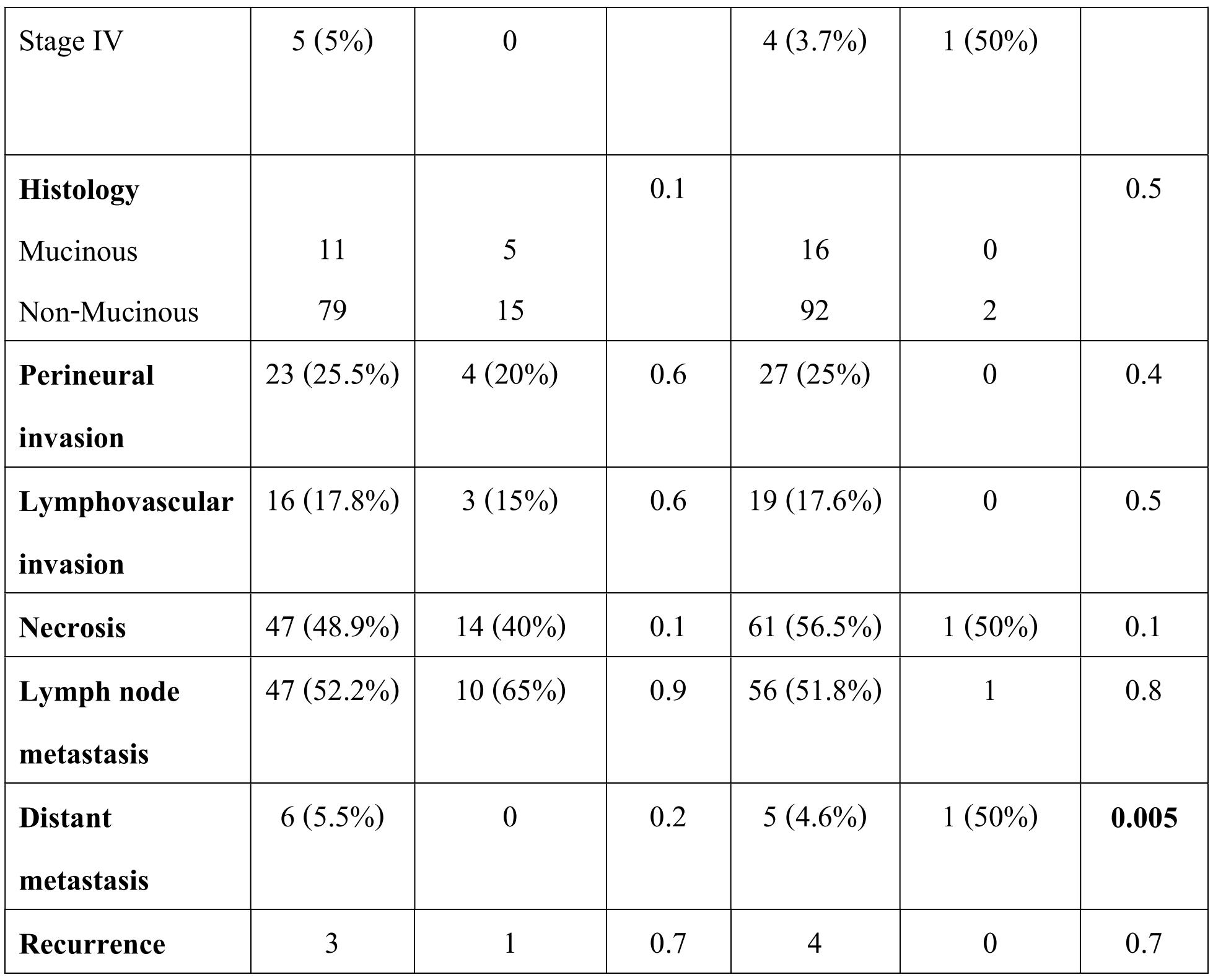
Table 3. Correlation of clinicopathological features with type of KRAS mutation status
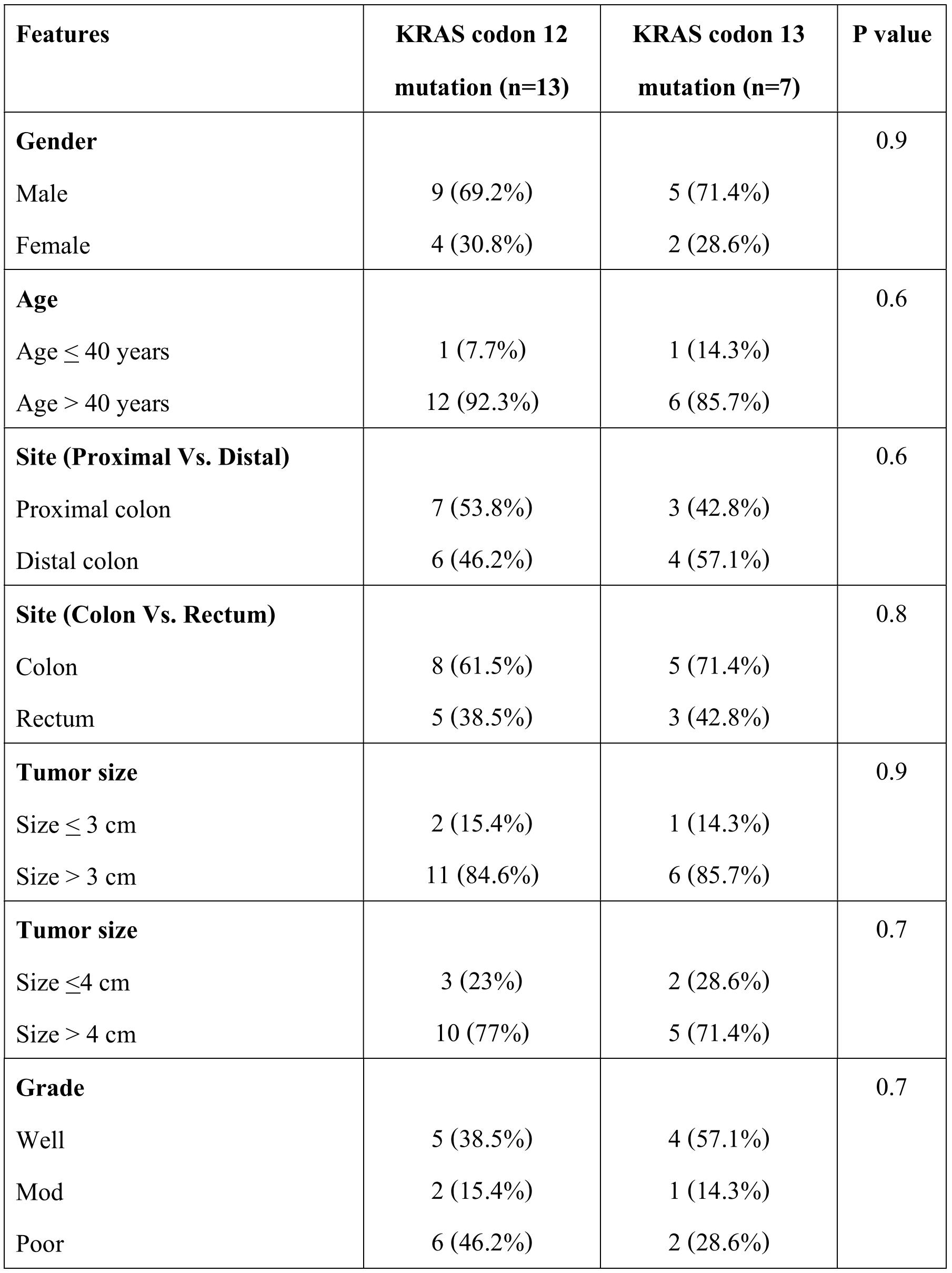
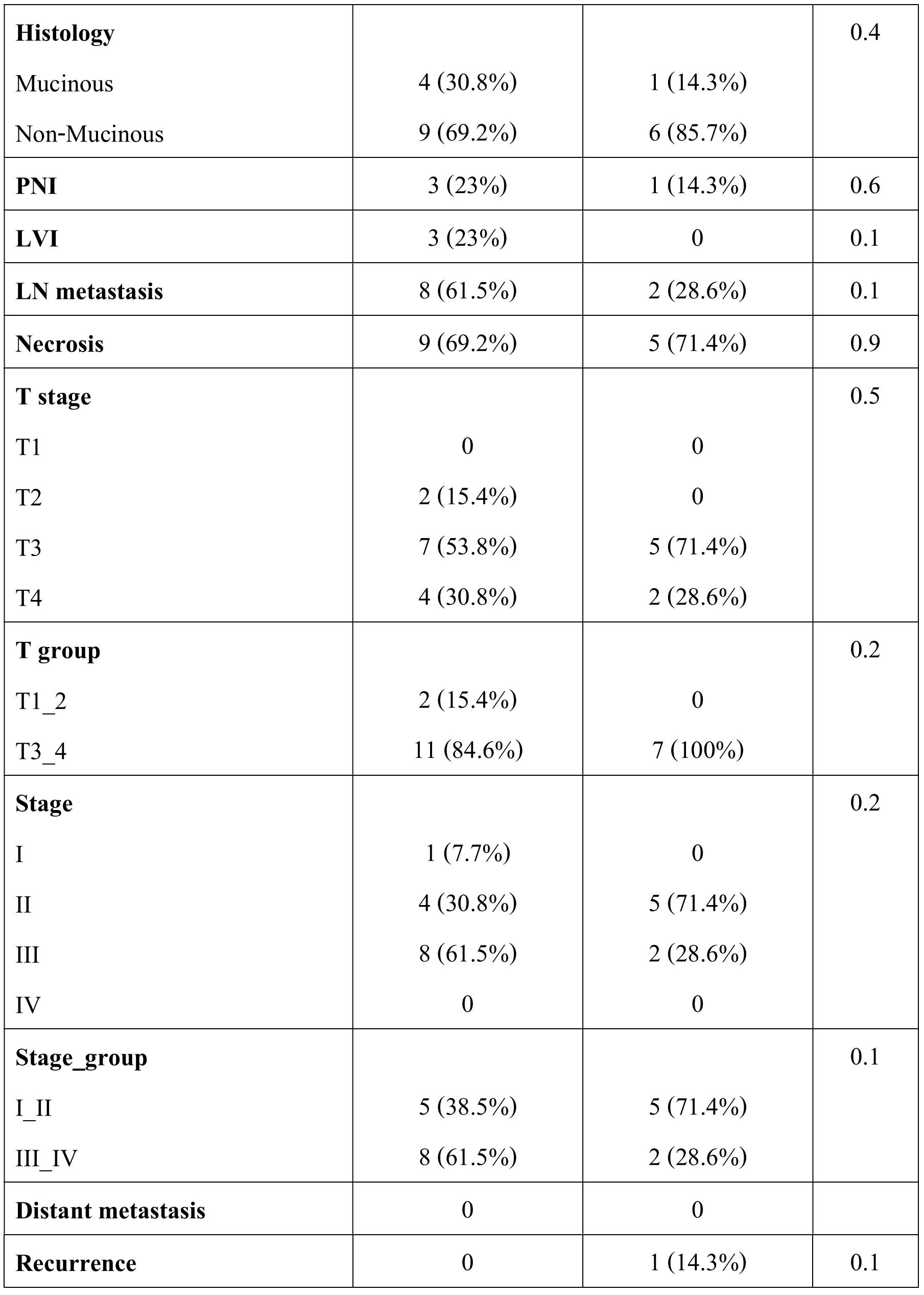
Table 4. Correlation of clinicopathological variables and KRAS - BRAF mutation status with age
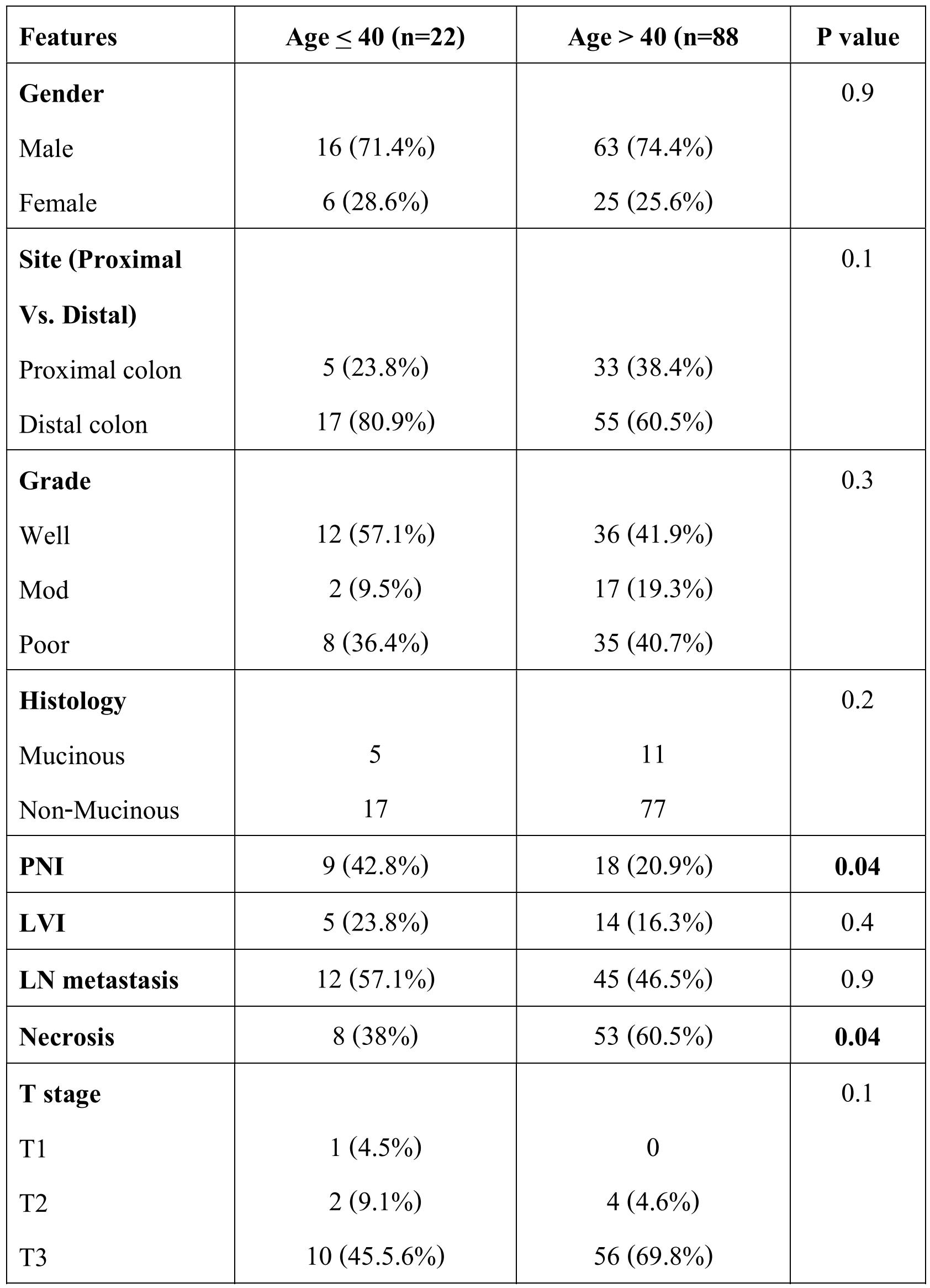
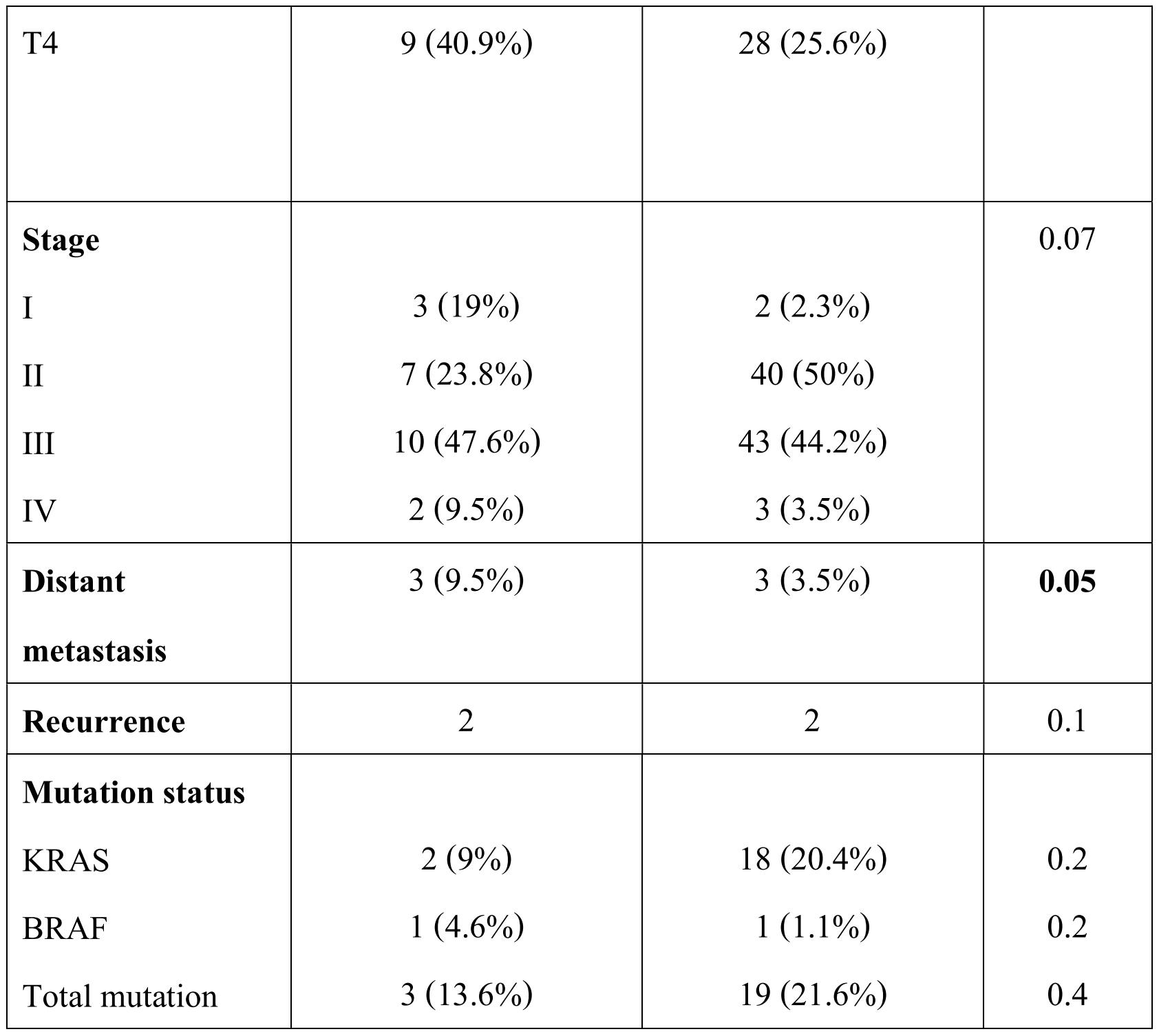
Table 5. Correlation of clinicopathological features and KRAS - BRAF mutation status with anatomic location
Discussion
Colorectal cancer is less common in Indians than the western population with an age adjusted incidence rates of 4.2 for males and 3.2 for females per 100000 populations as opposed to 35.3 and 25.7 per 100000 respectively in United States. Approximately 90% of CRC occurs in more than 50 years of age, however the incidence is increasing in younger patients of less than 40 years age in the recent past [15]. In the present study 20% of patients were less than 40 years of age. Targeted therapy with anti-EGFR monoclonal antibodies has been approved in metastatic (stage IV) CRC with better tolerance and improved survival. The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines recommend testing for KRAS mutations at the time of diagnosis of stage IV disease so as to better prepare an appropriate management plan in case treatment with an anti-EGFR antibody is later considered [16]. The KRAS mutation rate has been reported in the range of 22% to 40% [17-22] and that BRAF between 0% to 17% in different studies across the world [9,18,19,23-26]. The prevalence of KRAS mutation has been found to be lower in Indian patients as compared to other Asian studies and western population [20-22,27-29]. In the present study KRAS mutation was detected in 18.2% cases which are nearly similar to that reported in other Indian studies from north and west parts of India, [17-19] however the distribution of codon 12 and codon 13 mutations were almost similar to the previously reported studies. However, one study from south India showed KRAS mutation frequency of 46. 8% [30]. We did not find V600E mutation in our study, however two (1.8%) other reported mutations were found, the role of the same on prognosis is not well described in the literature because of its rarity. This is in contrast to that reported by Bagadi et al and Bisht et al from India [18,19] as well as from other Asian studies [28-31]. The mutation rate in BRAF varies widely even in the western population from 0 to 15% [23-25]. Low rate of KRAS mutations found in the present study are in accordance with those found from other parts of India,[17-19] however reason for low rate BRAF mutations along with absence of V600E is largely unexplained and should be supported or negated by carrying out larger studies in different parts of our country. One of the possible reasons could be variation in dietary habits and environmental factors in different parts of the country.
The association of KRAS and BRAF mutation is again variable in different studies. KRAS mutant cases and the type of mutation did not show any significant difference or association with respect to age, gender, anatomic location, histological features, metastatic disease and recurrence whereas one of the two BRAF mutant CRC showed association with advanced stage and distant metastasis (table 2). Sameer et al from Kashmir reported a significant association of KRAS mutation with advanced stage, lymph node metastasis and mucinous histology [17]. In contrast to this and similar to the present study, Bagadi et al did not show any association of KRAS, BRAF or NRAS mutation with clinicopathological features [18]. In another study from India by Bisht et al, KRAS and BRAF mutation was associated with age and tumor differentiation [19]. Yoshitake et al from Japan and Yuen et al from Honk Kong did not show any significant association of KRAS or BRAF mutation with age, gender, differentiation or location [26,31] except for association of BRAF mutation with lower Duke’s stage by Yien et al. In contrast to this, Lin et al from Taiwan showed significant association of KRAS and BRAF mutation with gender, location, differentiation and mucinous histology [28]. Birgisson et al from Sweden also showed significant association of KRAS and BRAF with stage and dissemination of CRC [27] Rosty et al from Australia also showed association of KRAS mutation with mucinous histology and proximal anatomic location [32] Similar reports have been published by Gunal et al from New York and Chang et al from California [33] In the present study though mutation status of KRAS and BRAF did not show any significant association with pathological factors, younger age (<40 years) at presentation was associated with higher perineural invasion (p=0.04), lower necrosis (p=0.04) and a marginally higher distant metastasis (p=0.05) (table 3). The proximal anatomic location also showed a significant association with tumor size of more than 3 cm (p=0.001), lower disease stage (p=0.04) and lower lymph node metastases (p=0.007) (table 4, 5). Thus all these evidences show that there is KRAS has a lower mutation rate in Indian population and BRAF mutation is also quite variable as evident in the studies by Bagadi et al (17%) and Bisht et al (9.8%) from India [18,19] These findings also suggest that KRAS and BRAF mutations have a variable effect on prognosis. Considering the role of anti-EGFR therapy in CRC, testing for potential biomarkers in order to recognize responders and nonresponders is now becoming important in the management of advanced or metastatic CRC. Data from different trials clearly demonstrates that patients with mutated KRAS are unlikely to benefit from anti-EGFR antibody therapy. However, it is not clear that patients with KRAS wild type will definitely respond, only that they have a reasonable opportunity to derive clinical benefit from the therapy. For example, in the panitumumab Vs. best supportive care trial, there were no responders in the mutated KRAS group randomized to the therapy and only a 17% partial response rate was noted in the KRAS wild-type group [34]
Moreover role of anti-EGFR therapy has been recommended in metastatic or advanced CRC. In the present study, according to the current NCCN guidelines [16] there were only 6 cases of metastatic CRC, which were candidates for anti-EGFR therapy and thus ideally suitable for KRAS-BRAF testing. In countries where most of the patients are of low socio-economic group, hardly 10% of metastatic CRC patients would afford for the anti-EGFR therapy and molecular biomarker testing. Going by the data of the present study, there would be one to two cases of CRC out of 110 cases that would need and may afford biomarker testing for anti-EGFR therapy. With limited resources, it would be preferable to start with KRAS mutation testing in metastatic disease followed by BRAF testing if KRAS is wild type. Although the current NCCN recommends extended RAS and BRAF with deficient mismatch repair / microsatellite instability testing, we suggest that exon 1 of KRAS and exon 15 of BRAF would be tested first, as these two exons cover nearly 90% [16,20,2325] of mutations occurring in these markers, followed by other exons of KRAS and NRAS and MSI testing in countries with low socio-economic group where health care burden is to borne by the patients themselves.
Conclusion
The present study shows a lower rate of KRAS mutations and rare BRAF mutations in CRC in Indian patients. However all the metastatic CRCs were wild type for KRAS except one which showed the rare BRAF mutation (p.R603L), significance of which is not well known. The younger age (<40 years) at presentation and anatomic location but not the mutation status of KRAS or BRAF showed significant association with tumor size, perineural invasion, necrosis, disease stage and lymph node metastasis. In countries with low socio-economic conditions, metastatic CRCs should be chosen judiciously for molecular biomarker testing and anti-EGFR therapy, preferably starting with common mutations first and then going to extended panel. This will reduce the extra burden on patients who can barely afford the minimum required treatment of their advanced disease.
Conflict of interest: None
References:
1. K. M. Mohandas. Colorectal cancer in India: controversies, enigmas and primary prevention. K. M. Mohandas. Indian J Gastroenterol 2011;30:3-6.
2. Curado MP, Edwards B, Shin HR, Ferlay J, Heanue M, Boyle P. (Eds). Cancer Incidence in five continents, Volume IX. IARC Scientific Publication, No 160 IARC, Lyon 2007.
3. National Cancer Registry Programme. Accessed at: http://www.icmr.nic.in/ncrp/cancer_reg. htm on 15th October, 2016.
4. Wolpin BM, Mayer RJ: Systemic treatment of colorectal cancer. Gastroenterology 2008; 134:1296-10.
5. Jean GW, Shah SR. Epidermal growth factor receptor monoclonal antibodies for the treatment of colorectal cancer. Pharmacotherapy 2008;28:742-4.
6. Peeters M, Balfour J, Arnold D. Panitumumab—a fully human anti-EGFR monoclonal antibody for treatment of metastatic colorectal cancer. Aliment Pharmacol Ther. 2008;28:269-1.
7. Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27:1130-6.
8. Lea IA, Jackson MA, Li X, Bailey S, Peddada SD, Dunnick JK. Genetic pathways and mutation profiles of human cancers: site- and exposure-specific patterns. Carcinogenesis. 2007;28:1851-8.
9. Lie'vre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5.
10. De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectalcancer treated with cetuximab. Ann Oncol 2008;19:508-5 .
11. Wilson PM, Labonte MJ, Lenz HJ. Molecular markers in the treatment of metastatic colorectal cancer. Cancer J. 2010;16:262-2.
12. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-4.
13. Nelson R. Anti-EGFR therapy worsens survival in patients with RAS mutations. Medscape Medical News. September 11, 2013. Available at http://www.medscape.com/viewarticle/8 10817. Last Accessed: 15th October, 2016.
14. Berlin J. Beyond exon 2--the developing story of RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1059-60.
15. Sudarshan V, Hussain N, Gahine R, Mourya J. Colorectal cancer in young adults in a tertiary care hospital in Chhattisgarh, Raipur. Indian J Cancer 2013;50:337-40.
16. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Colon Cancer V.3.2012. National Comprehensive Cancer Network. Available at http://www.nccn.org/professionals/physi cian_gls/PDF/colon.pdf. Last Accessed: 15th October, 2016.
17. Sameer AS, Chowdhri NA, Abdullah S, Shah ZA, Siddiqi MA. Mutation pattern in K-ras gene in colorectal cancer patients of Kashmir: A report. Indian J Cancer 2009;46:219-25.
18. Bagadi SB, Sanghvi M, Nair SB, Das BR. Combined mutational analysis of KRAS, NRAS and BRAF genes in Indian patients with colorectal carcinoma. Int J Biol Markers. 2012;27:2733.doi: 10.5301/JBM.2012.9108.
19. Bisht S, Ahmad F, Sawaimoon S, Bhatia S, Das BR. Molecular spectrum of KRAS, BRAF, and PIK3CA gene mutation: determination of frequency, distribution pattern in Indian colorectal carcinoma. Med Oncol. 2014^31:124. doi: 10.1007/s1203201401243.
20. Plesec TP, Hunt JL. KRAS Mutation Testing in Colorectal Cancer. Adv Anat Pathol 2009;16:196-203.
21. Monzon FA, Ogino S, Hammond ME, Halling KC, Bloom KJ, et al. The Role of KRAS Mutation Testing in the Management of Patients with Metastatic Colorectal Cancer. Arch Pathol Lab Med. 2009;133:1600-6.
22. Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193-7.
23. Khambata-Ford S1, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230-7.
24. Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255-9.
25. Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M, et al. Detection of BRAF V600E mutation by pyrosequencing. Pathology. 2008;40:295-8.
26. Yoshitake N, Fujii S, Mukawa K, Tominaga K, Fukui H, Ichikawa K, et al. Mutational analysis of the BRAF gene in colorectal mucinous carcinoma in association with histological configuration. Oncol Rep. 2007;17:9-15.
27. Kawazoe A, Shitara K, Fukuoka S, Kuboki Y, Bando H, Okamoto W, et al. A retrospective observational study of clinicopathological features of KRAS, NRAS, BRAF and PIK3CA mutations in Japanese patients with metastatic colorectal cancer. BMC Cancer 2015;15:258.DOI 10.1186/s12885-015-1276-z.
28. Lin CC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, et al. The Prognostic Role of Microsatellite Instability, Codon-Specific KRAS, and BRAF Mutations in Colon Cancer. Journal of Surgical Oncology 2014;110:451-7.
29. Hsieh LL, Er TK, Chen CC, Hsieh JS, Chang JG, Liu TC. Characteristics and prevalence of KRAS, BRAF, and PIK3CA mutations in colorectal cancer by high resolution melting analysis in Taiwanese population. Clin Chim Acta. 2012;413:1605-11.
30. Veldore VH, Rao MR, Prabhudesai SA, Tejaswi R, Kakara S, Pattanayak S, Krishnamoorthy N, Tejaswini BN, Hazarika D, Gangoli A, Rahman SM,
32. Dixit J, Naik R, Diwakar RB, Satheesh CT, Shashidhara HP, Patil S, Gopinath KS, Ajai Kumar BS. Prevalence of KRAS mutations in metastatic colorectal cancer: A retrospective observational study from India. Indian J Cancer 2014;51:531-7.
31. Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res 2002;62:6451-5.
32. Rosty C, Young JP, Walsh MD, Clendenning M, Walters RJ, Pearson S, et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013. doi: 10.1038/modpathol.2012.240.
33. Gunal A, Hui P, Kilic S, Xu R, Jain D, Mitchell K, et al. KRAS Mutations are Associated with Specific Morphologic Features in Colon Cancer. J Clin Gastroenterol. 2012;47:509-14.
34. Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-34.


